navneet9431
see this diagram of the structure of diamond given in my textbook-
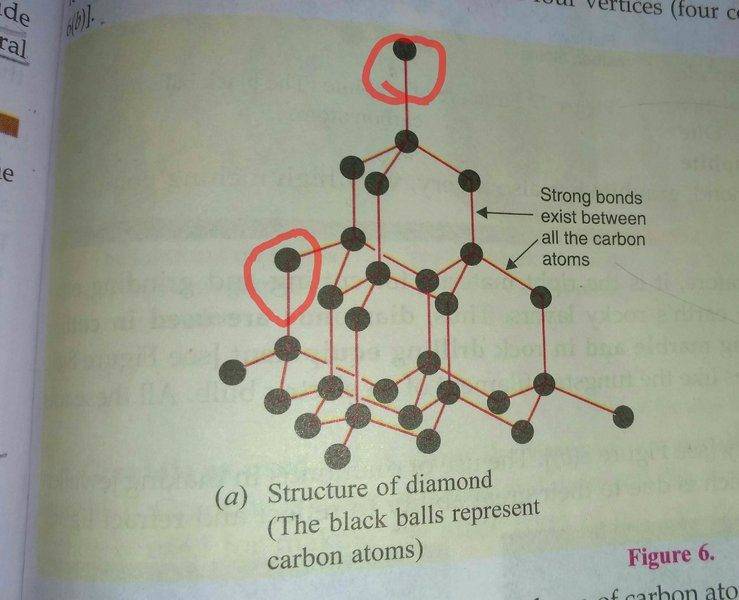
I have read in my textbook that each carbon atom in a Diamond crystal is attached to four other carbon atoms by strong covalent bonds.But, in the diagram above I can see that some of the carbon atoms are not attached to 4 carbon atoms(see the red circle).
So, I want to know, Is this diagram of the structure of Diamond incorrect?
If not, then please explain to me why?
I will be thankful for any help!
Note: I am a high school student and English is my second language.
I have read in my textbook that each carbon atom in a Diamond crystal is attached to four other carbon atoms by strong covalent bonds.But, in the diagram above I can see that some of the carbon atoms are not attached to 4 carbon atoms(see the red circle).
So, I want to know, Is this diagram of the structure of Diamond incorrect?
If not, then please explain to me why?
I will be thankful for any help!
Note: I am a high school student and English is my second language.