SUMMARY
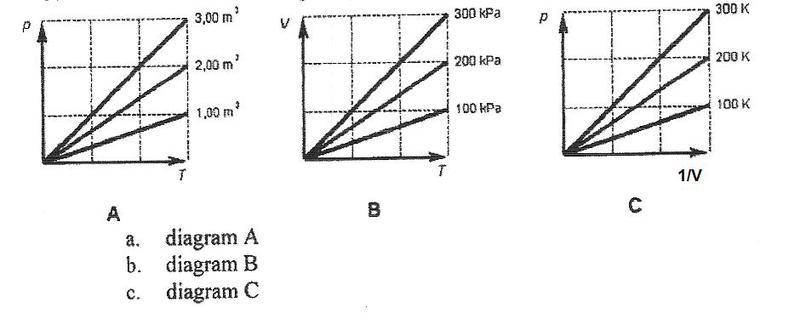
The discussion centers on identifying the correct diagram that represents the behavior of an ideal gas, specifically diagram C. The ideal gas law, expressed as PV = nRT, is fundamental to understanding the relationships between pressure (P), volume (V), and temperature (T). The participants clarify that diagram C accurately illustrates how a larger temperature results in a steeper slope, while diagrams A and B incorrectly depict the relationships between the variables. The key takeaway is that understanding the slopes in relation to the ideal gas law is essential for interpreting these diagrams correctly.
PREREQUISITES
- Understanding of the Ideal Gas Law (PV = nRT)
- Familiarity with graph interpretation in physics
- Knowledge of the relationships between pressure, volume, and temperature
- Basic algebra for manipulating equations
NEXT STEPS
- Study the derivation and applications of the Ideal Gas Law (PV = nRT)
- Learn about graphical representations of gas laws and their slopes
- Explore the implications of changing temperature on gas behavior
- Investigate real gas behavior versus ideal gas behavior
USEFUL FOR
This discussion is beneficial for physics students, educators, and anyone interested in thermodynamics and gas laws, particularly those studying the behavior of ideal gases in various conditions.