- #1
pureouchies4717
- 99
- 0
HI! i was wondering if someone could please help me with this physics problem. it looks very simple, and I am only missing one value. thanks
gas pressure is: p= F/A = (mg)/A(of the cylinder)
my problem is: how can i get the mass of the piston? its really annoying me. please help. thanks
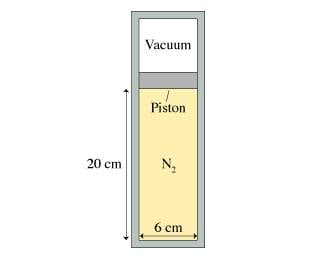
A 6.0-cm-diameter cylinder of nitrogen gas has a 4.0-cm-thick movable copper piston. The cylinder is oriented vertically, as shown in the figure, and the air above the piston is evacuated. When the gas temperature is 20 degree C, the piston floats 20 cm above the bottom of the cylinder.What is the gas pressure?
gas pressure is: p= F/A = (mg)/A(of the cylinder)
my problem is: how can i get the mass of the piston? its really annoying me. please help. thanks