RJLiberator
Gold Member
- 1,094
- 63
Okay, I am in need of some guidance:
7)Calculate the wavelength that corresponds to an emission of energy of 1.977x10^-19 J.
Okay, well here is my thought process initially: Change in Energy = hc/wavelength.
First question: Is 1.977*10^-19 THE change in energy? OR is that the Energy FINAL and the Energy initial is some constant that I SHOULD know already?
If it is the CHANGE in energy then using the equation I can get the wavelength rather easy by simply plugging in Planck's constant and the speed of light. My answer was 1005 nm doing this process.
My question to YOU is is this correct? OR am I missing the initial energy, and if so, where can I find this 'initial energy?'
Next,
8)If the initial energy level for the energy change in problem seven is n = 7, what is the final energy level?
Uhhh.. how do I find this? Any leads on this? I feel like I am missing some chart of numbers that corresponds the energy changes to certain "n" levels.
And finally,
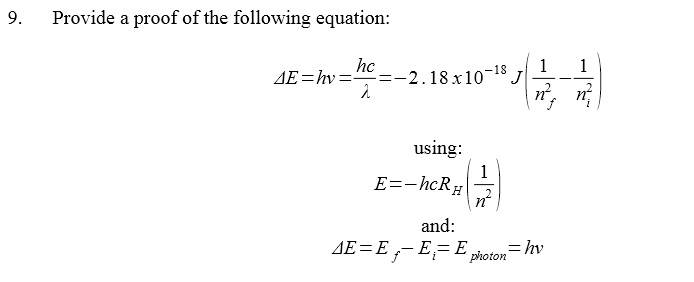
Any leads, websites, directions I can check out to get me started here?
Thanks all for your help.
7)Calculate the wavelength that corresponds to an emission of energy of 1.977x10^-19 J.
Okay, well here is my thought process initially: Change in Energy = hc/wavelength.
First question: Is 1.977*10^-19 THE change in energy? OR is that the Energy FINAL and the Energy initial is some constant that I SHOULD know already?
If it is the CHANGE in energy then using the equation I can get the wavelength rather easy by simply plugging in Planck's constant and the speed of light. My answer was 1005 nm doing this process.
My question to YOU is is this correct? OR am I missing the initial energy, and if so, where can I find this 'initial energy?'
Next,
8)If the initial energy level for the energy change in problem seven is n = 7, what is the final energy level?
Uhhh.. how do I find this? Any leads on this? I feel like I am missing some chart of numbers that corresponds the energy changes to certain "n" levels.
And finally,
Any leads, websites, directions I can check out to get me started here?
Thanks all for your help.