astralfx
- 22
- 0
I was just bored during a lecture and started dozing of, then I had an idea of how heat travels. I tried searching in google if I was right, but I didn't know what to type (I tried stuff to do with second law of thermo, and IR photons traveling but couldn't find anything).
So yeah I thought I'd ask you lot before the physicsforum because I cba to find my login/pass, when atoms vibrate/move/rotate it emits heat radiation (IR photon), what my original thought was how can these randomly emitted IR photons at random angles travel to the cooler region.
That's when it hit me, is it because when IR photons are produced and come in contact with another atom, "all or nothing" law occurs, so either the IR photon is absorbed by the neighbouring atom or it isn't, and if it isn't or the IR photon is in the ER field of the atom, it causes the photon to be repelled by the atoms ER field thus changing the angle of the IR photon. Because the cooler region, well between the cool and hot region is a gradient of hot -> cool, thus atoms are more likely to absorb the IR photons between the gradient rather than anywhere else eventually levelling the energy of all atoms in the gradient, thus for all the deflections occurring from the IR photon to atoms, it's more likely to travel through the gradient because it will end up reaching there even if it didn't start at those atoms because other atoms NOT in the gradient are most likely at there minimum energy needs so no need to absorb any more energy.
Sorry for the bad explanation, the image is sort of what I mean. Am I correct or ...?
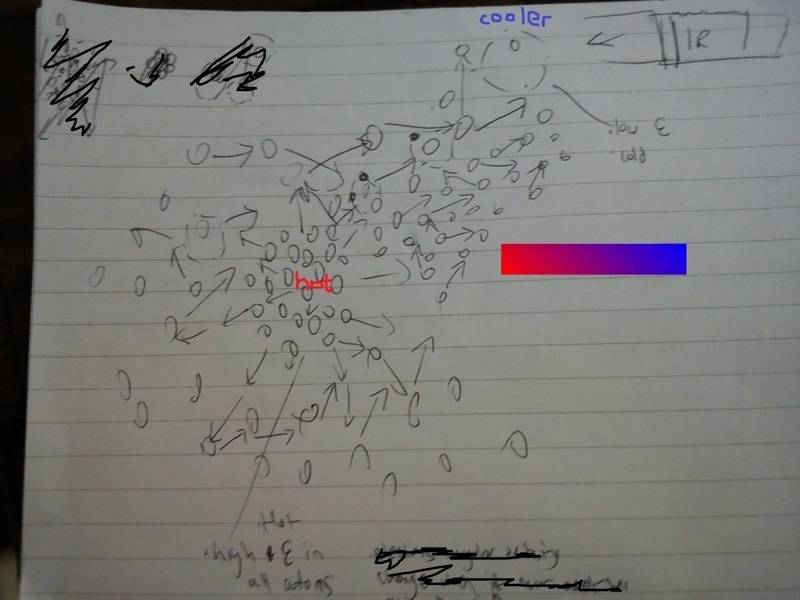
So yeah I thought I'd ask you lot before the physicsforum because I cba to find my login/pass, when atoms vibrate/move/rotate it emits heat radiation (IR photon), what my original thought was how can these randomly emitted IR photons at random angles travel to the cooler region.
That's when it hit me, is it because when IR photons are produced and come in contact with another atom, "all or nothing" law occurs, so either the IR photon is absorbed by the neighbouring atom or it isn't, and if it isn't or the IR photon is in the ER field of the atom, it causes the photon to be repelled by the atoms ER field thus changing the angle of the IR photon. Because the cooler region, well between the cool and hot region is a gradient of hot -> cool, thus atoms are more likely to absorb the IR photons between the gradient rather than anywhere else eventually levelling the energy of all atoms in the gradient, thus for all the deflections occurring from the IR photon to atoms, it's more likely to travel through the gradient because it will end up reaching there even if it didn't start at those atoms because other atoms NOT in the gradient are most likely at there minimum energy needs so no need to absorb any more energy.
Sorry for the bad explanation, the image is sort of what I mean. Am I correct or ...?