SUMMARY
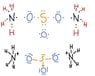
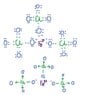
The discussion focuses on drawing Lewis structures for the compounds (NH4)2SO3, Al2(SO4)3, and Fe(ClO4)3. Participants share their attempts, particularly highlighting challenges with Al2(SO4)3. The consensus is that while the structures for (NH4)2SO3 and Fe(ClO4)3 can be logically deduced, Al2(SO4)3 requires a more nuanced understanding of sulfate ion bonding and resonance. Accurate Lewis structures are essential for predicting molecular geometry and reactivity.
PREREQUISITES
- Understanding of Lewis structures and dot structures
- Knowledge of ionic and covalent bonding
- Familiarity with polyatomic ions, specifically sulfate (SO4)2-
- Basic principles of molecular geometry and resonance
NEXT STEPS
- Research the Lewis structure of sulfate ions (SO4)2-
- Learn about resonance structures and their significance in molecular stability
- Study the molecular geometry of Al2(SO4)3 using VSEPR theory
- Explore the properties and bonding of transition metal compounds like Fe(ClO4)3
USEFUL FOR
Chemistry students, educators, and anyone involved in molecular structure analysis will benefit from this discussion.