- #1
AAMAIK
- 47
- 0
Diffusion of A through Non-diffusing B
Dry air is required for burning of sulphur in a sulphuric acid plant. Moisture (A) diffuses through a film or layer of air (B), reaches the acid and gets absorbed in it. But air being virtually insoluble in sulphuric acid will not diffuse. So air is non-diffusing.
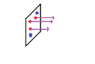
If we consider steady-state molecular diffusion through a constant area. A diffuses because it has a non zero gradient of partial pressure at each point of the diffusion path. But Why is it so that B (Blue circles) is not diffusing if at each point along the diffusion length its partial pressure varies due to the constant outflux of molecules A (red circles) in the direction of decreasing concentration or partial pressure?
Dry air is required for burning of sulphur in a sulphuric acid plant. Moisture (A) diffuses through a film or layer of air (B), reaches the acid and gets absorbed in it. But air being virtually insoluble in sulphuric acid will not diffuse. So air is non-diffusing.
If we consider steady-state molecular diffusion through a constant area. A diffuses because it has a non zero gradient of partial pressure at each point of the diffusion path. But Why is it so that B (Blue circles) is not diffusing if at each point along the diffusion length its partial pressure varies due to the constant outflux of molecules A (red circles) in the direction of decreasing concentration or partial pressure?