willloomy
- 4
- 0
hey everybody, I need to figure out a few things about calculating the pressure inside a vacuum
basically I have a tube and one side of the tube is capped and air tight and the other side is open to the air. In the tube there is an air tight piston like stopper that is pulled through the tube towards open end of the tube, creating a vacuum on the opposite side of the piston.
I need to figure out how much force is needed to pull the piston various distances in the tube.
I have a 1 inch inner diameter tube right now but I would like to be able to test different sized tubes.
hope that wasn't to confusing, hope this picture helps.
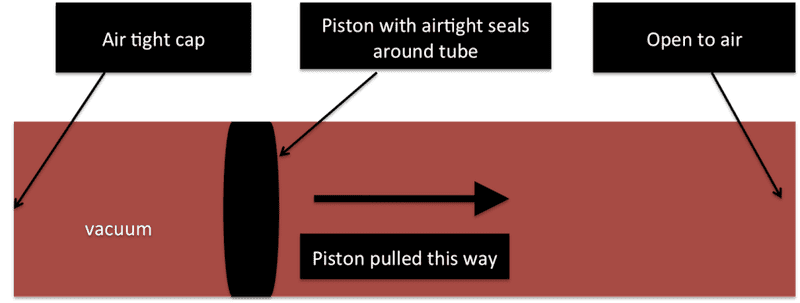
basically I have a tube and one side of the tube is capped and air tight and the other side is open to the air. In the tube there is an air tight piston like stopper that is pulled through the tube towards open end of the tube, creating a vacuum on the opposite side of the piston.
I need to figure out how much force is needed to pull the piston various distances in the tube.
I have a 1 inch inner diameter tube right now but I would like to be able to test different sized tubes.
hope that wasn't to confusing, hope this picture helps.