Discussion Overview
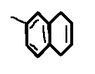
The discussion revolves around the Diels-Alder reaction involving p-methylbenzyn and 1,3-butadien. Participants explore the identity of the reactants and the expected product of the reaction.
Discussion Character
- Homework-related, Technical explanation
Main Points Raised
- One participant poses a homework question regarding the product of the reaction between p-methylbenzyn and 1,3-butadien.
- Another participant questions the nomenclature of "p-methylbenzyn," suggesting it does not make sense.
- A different participant proposes that "3-methylbenzyn" (3-methyldehydrobenzen) might be the intended compound.
- One participant suggests that a benzyne intermediate is likely involved and asks for clarification on which compound serves as the diene and which as the dienophile.
- There is agreement that the dienophile is methylbenzyn and the diene is 1,3-butadien, with one participant stating that determining the product is a straightforward application of the Diels-Alder mechanism.
- Participants express positive reinforcement towards each other’s contributions.
Areas of Agreement / Disagreement
There is some confusion regarding the correct nomenclature of the reactants, and while participants agree on the roles of the diene and dienophile, the discussion does not reach a consensus on the identity of the starting materials.
Contextual Notes
Participants have not resolved the ambiguity surrounding the naming of "p-methylbenzyn," and the discussion relies on the assumption that the Diels-Alder mechanism applies to the proposed reaction.
Who May Find This Useful
Students studying organic chemistry, particularly those interested in reaction mechanisms and nomenclature in organic compounds.