Phoenixtears
- 82
- 0
SOLVED
1. Homework Statement
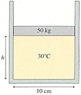
A circular disc of lead has a mass 50 kg, it acts as the piston of a circular container shown in Figure CP16.69. The disc floats on 0.140 mol of compressed air. (Image Attached)
Figure CP16.69
(a) What is the piston height h if the temperature is 30°C?
cm
(b) How far does the piston move if the temperature is increased by 105°C?
cm
The following questions involve work, recall conditions for (-) work and (+) work ...
(c) How much work did the atmosphere do on the piston?
Nm
(d) How much work did the gravity field do on the lead piston
Nm
(e) How much work did the gas do on the lead piston?
Nm
P= F/A
PV=nRT
I haven't tried anything but A- I'm stuck on that one.
I first found the pressure using P=F/a. I found the weight by doing 50*9.8m/s2 and then divided by (pi)*r^2. That answer, which is already in Pa, I then added to 101,300 pa, the atmospheric constant for pressure. That pressure I used in PV= nRT to find volume. Then I used the volume of a cylinder formula (V= (pi)*r^2*h) to find height. This answer did not come out right (and I made sure to be in centimeters). Does anyone know where I'm going wrong?
Thanks in advance!
~Phoenix
1. Homework Statement
A circular disc of lead has a mass 50 kg, it acts as the piston of a circular container shown in Figure CP16.69. The disc floats on 0.140 mol of compressed air. (Image Attached)
Figure CP16.69
(a) What is the piston height h if the temperature is 30°C?
cm
(b) How far does the piston move if the temperature is increased by 105°C?
cm
The following questions involve work, recall conditions for (-) work and (+) work ...
(c) How much work did the atmosphere do on the piston?
Nm
(d) How much work did the gravity field do on the lead piston
Nm
(e) How much work did the gas do on the lead piston?
Nm
Homework Equations
P= F/A
PV=nRT
The Attempt at a Solution
I haven't tried anything but A- I'm stuck on that one.
I first found the pressure using P=F/a. I found the weight by doing 50*9.8m/s2 and then divided by (pi)*r^2. That answer, which is already in Pa, I then added to 101,300 pa, the atmospheric constant for pressure. That pressure I used in PV= nRT to find volume. Then I used the volume of a cylinder formula (V= (pi)*r^2*h) to find height. This answer did not come out right (and I made sure to be in centimeters). Does anyone know where I'm going wrong?
Thanks in advance!
~Phoenix
Attachments
Last edited: