- #1
physicsdoc
- 1
- 0
Hello, I need help with 2 homework questions:
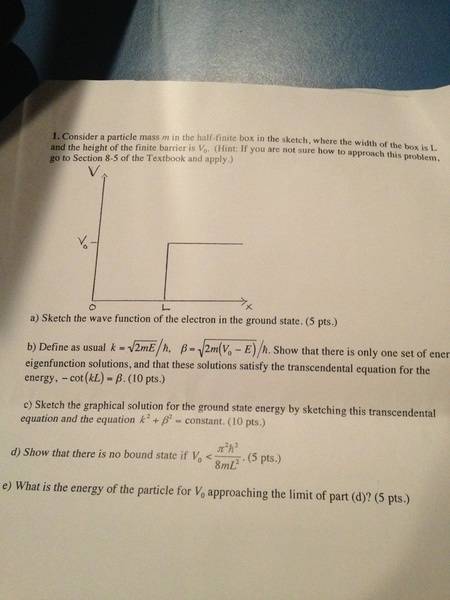
Also this question:

Also this question:
Bounded states in quantum mechanics refer to the energy states of a system where the energy is finite and confined within a certain range. These states are characterized by having a wavefunction that approaches zero as the distance from the center of the system increases.
Bounded states have finite energy and are confined within a certain range, while unbounded states have infinite energy and are not confined to a specific region. Bounded states also have discrete energy levels, while unbounded states have a continuous spectrum of energy.
Bounded states play a crucial role in understanding the behavior of quantum systems, as they represent the stable energy states that particles can occupy. These states also determine the possible energy levels that a system can have and the transitions between them.
In quantum mechanics, bounded states are described by the Schrödinger equation, which is a differential equation that relates the wavefunction of a particle to its energy. The wavefunction is used to calculate the probability of finding a particle in a particular location within the system.
Yes, bounded states can exist in all quantum systems, including atoms, molecules, and subatomic particles. However, the number and energy levels of bounded states vary depending on the specific system and its properties.