small physicist
- 3
- 0
Hello,
If I shine a red laser onto a white surface, I assume that:
I know that my laser has this extremely narrow spectrum:
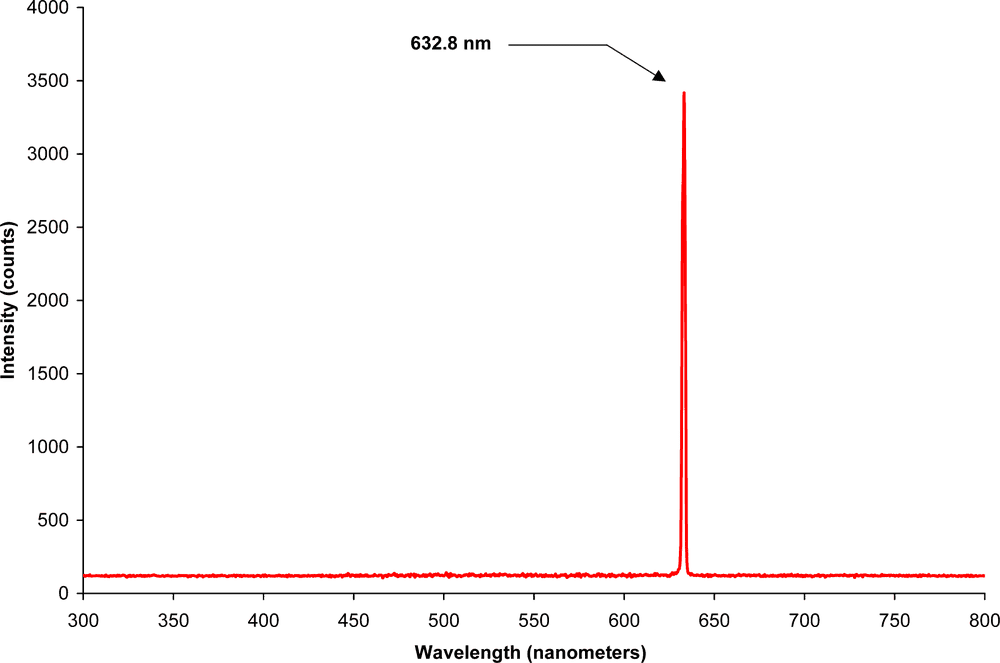
but what spectrum does a white surface emit when I shine it with said laser?
Thanks in advance.
If I shine a red laser onto a white surface, I assume that:
- some of the red light will be nearly instantly reflected,
- some will be absorbed and converted into phonons
- and some will be absorbed and re-emitted.
I know that my laser has this extremely narrow spectrum:
but what spectrum does a white surface emit when I shine it with said laser?
Thanks in advance.
