randomgamernerd
- 139
- 4
I have uploaded the picture of the structures because I couldn't figure out how to type.
Its vinyl chloride.
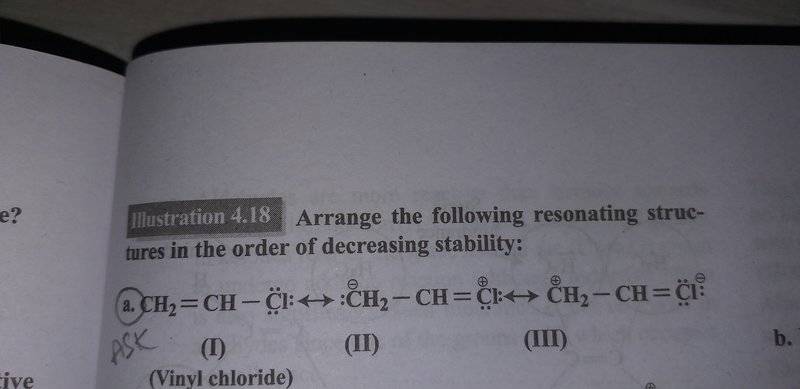
According to me the order should be
I>III>II
because I has no charge so it must be most stable
in II we have Cl an electronegative element bearing positive charge which makes it unstable.
Hence I get the order.
But the book says the order is I>II>III
Please Help
Its vinyl chloride.
According to me the order should be
I>III>II
because I has no charge so it must be most stable
in II we have Cl an electronegative element bearing positive charge which makes it unstable.
Hence I get the order.
But the book says the order is I>II>III
Please Help