cookiemnstr510510
- 162
- 14
Hello All,
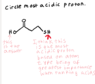
I have attached my questions with pictures in attached photos. Look at screen shot 46 first then screen shot 47.
I am having trouble ranking acidic protons.
In my Organic book it has the order of:
1)Charge
2)Atom Type
3)Hybridization
4)Resonance
5)Induction
Things I know:
S is able to accommodate the negative charge after deprotonation better than O due to size.
I also see that if O is deprotonated that the negative charge is able to go through resonance which would stabilize the conjugate base better.
If the list shows Atom type being more important than resonance, why is the H on the O the most acidic proton?
The only thing I can think of is that O and S are not too different in terms of being able to hold charge (We already know that S can accommodate the negative charge better), but since O has another property from the list that S doesn't then O wins because the effects are additive?
Very confused.
I know if you look at the PKa's that OH has a lower PKa than SH. I want to understand this on a more fundamental level rather than just PKa.
Thanks,
WIll
I have attached my questions with pictures in attached photos. Look at screen shot 46 first then screen shot 47.
I am having trouble ranking acidic protons.
In my Organic book it has the order of:
1)Charge
2)Atom Type
3)Hybridization
4)Resonance
5)Induction
Things I know:
S is able to accommodate the negative charge after deprotonation better than O due to size.
I also see that if O is deprotonated that the negative charge is able to go through resonance which would stabilize the conjugate base better.
If the list shows Atom type being more important than resonance, why is the H on the O the most acidic proton?
The only thing I can think of is that O and S are not too different in terms of being able to hold charge (We already know that S can accommodate the negative charge better), but since O has another property from the list that S doesn't then O wins because the effects are additive?
Very confused.
I know if you look at the PKa's that OH has a lower PKa than SH. I want to understand this on a more fundamental level rather than just PKa.
Thanks,
WIll