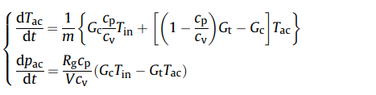AmeenBassam
- 2
- 0
Hello All,
I am trying to model an air vessel with a constant volume during charging and discharging with compressed air. Are the equations in the attached photo are suitable?
where m is the air mass, Gc and Gt are the mass flow rate in and out of the air vessel with a volume V. Tac is the temperature of the Air in air storage device.
I am trying to model an air vessel with a constant volume during charging and discharging with compressed air. Are the equations in the attached photo are suitable?
where m is the air mass, Gc and Gt are the mass flow rate in and out of the air vessel with a volume V. Tac is the temperature of the Air in air storage device.