Hatyk
- 6
- 0
Homework Statement
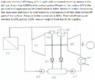
Calculate thermal efficiency of R-C cycle with saturated steam as shown in the picture. Steam has 4,5MPa when entering into HP part of the turbine (VT in the picture) and expansion on HP is finished at 0,3MPa. Moisture is then removed in the separator and steam is overheated to a temperature 8K less then before HP part of the turbine. Pressure in the condenser is 4kPa. Thermal efficiency of turbines is 85%, pumps 100%. You can neglect heat loss in the pipeline.
[/B]
Homework Equations
Hello, I've encountered a problem with this cycle. I seem to miss value of the pressure in the deaerator (NN in the picture). Is there a way to calculate the pressure or calculate enthalpy without this pressure?[/B]
The Attempt at a Solution
I was calculating the enthalpy values for the thermal efficiency, but the absence of pressure in the deaerator stopped me in my tracks. I was able to calculate enthalpy after both parts of the turbine, but got stuck at a point 5, right after condensate pump. To calculate point 5, I need pressure in the deaerator. If I knew the pressure I would be able to calculate enthalpy after the deaerator and with this enthalpy, I would be able to calculate enthalpy after the condensate pump using the binding equation from the deaerator. [/B]