SWJ
- 5
- 0
Hi,
I am currently studying Thermodynamics and stumbled upon this equation and is slightly confused as to how this works. Hopefully someone can help me with the understanding.
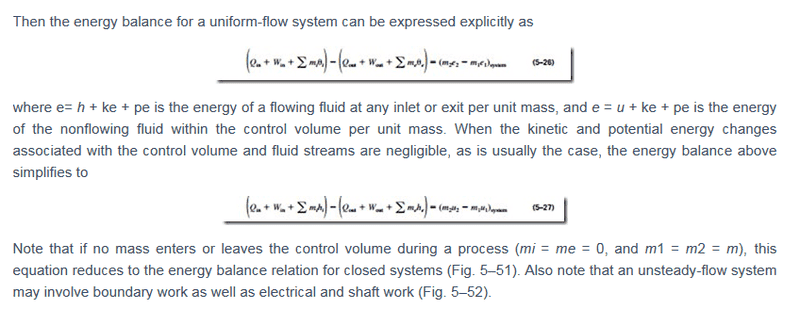
According to this text here, if I am not wrong e can be h + ke + pe or u + ke + pe depending on where i am looking the system at. So does that mean the final equation can be equal to (m1u1 - m2u2) or (m1h1 - m2h2) depending on where i am looking the system at? Or i should just ask when would it be mh rather than mu?
Thank you very much.
I am currently studying Thermodynamics and stumbled upon this equation and is slightly confused as to how this works. Hopefully someone can help me with the understanding.
According to this text here, if I am not wrong e can be h + ke + pe or u + ke + pe depending on where i am looking the system at. So does that mean the final equation can be equal to (m1u1 - m2u2) or (m1h1 - m2h2) depending on where i am looking the system at? Or i should just ask when would it be mh rather than mu?
Thank you very much.