Discussion Overview
The discussion revolves around the comparison of vaporization heat and heat capacity of water (H2O), specifically addressing the energy required to evaporate water at different temperatures and the implications for heating water to 100°C from 25°C. The scope includes theoretical and conceptual aspects of thermodynamics related to phase changes and heat transfer.
Discussion Character
- Technical explanation
- Conceptual clarification
- Debate/contested
Main Points Raised
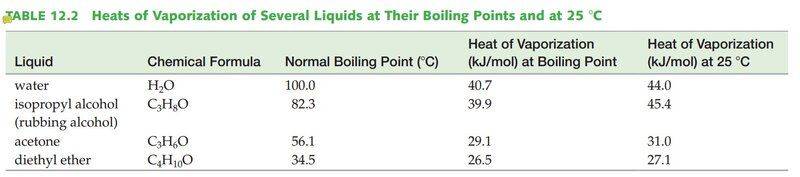
- One participant notes that the heat needed for 1 mol of H2O to evaporate at 100°C is 40.7 kJ, while at 25°C it is 44.0 kJ, leading to a calculation suggesting that 3.7 kJ is required to heat H2O to 100°C from 25°C.
- Another participant challenges this calculation, stating that to convert liquid water at 25°C to vapor at 100°C, one must heat the water and evaporate it or evaporate the water and heat the vapor, while also needing to do work to compress the vapor due to pressure differences.
- A participant expresses confusion about the process of evaporating water at 25°C and heating it, questioning whether lowering the pressure is involved and what compression work is necessary.
- Further clarification is provided that water can evaporate at all temperatures until the vapor reaches saturation, and that converting saturated vapor from 25°C to that at 100°C requires both heating and compression.
Areas of Agreement / Disagreement
Participants express differing views on the calculations and processes involved in heating and evaporating water, indicating that the discussion remains unresolved with multiple competing perspectives on the thermodynamic processes.
Contextual Notes
There are limitations in the assumptions made regarding the energy calculations and the need for work in compressing vapor, as well as the dependence on specific definitions of heat capacity and vaporization heat.