- #1
merbear
- 12
- 0
1. Homework Statement
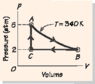
A 1 mol sample of an ideal gas for which c'V = 3R/2 follows the closed thermodynamic cycle shown in Fig. 18-36. There are three legs in the cycle: an isothermal expansion, A B; an isobaric compression, B C; and a constant-volume increase in pressure, C A. The temperature during the isothermal leg is 340 K, pA = 1.8 atm, and pB = 1.2 atm. Find each of the following. (a) Va (b) Vb. (See attachment for image - ignore the pressure numbers on the graph and use the numbers given in the problem)
2. Homework Equations
Pv=nRT (initial and final)
3. The Attempt at a Solution
Pa: V= [1 mol (.08205784 L*atm/k*mol)(340K)]/1.8 atm = 15.499 L = .0155 M^3
Pb: V= [1 mol (.08205784 L*atm/k*mol)(340K)]/`1.2 atm = 23.25 L = .0232 M^3
When I plugged those answers to see if they were right, the first one came up right for the volume at A. But my response for the volume at B came up incorrect. I performed the problem in the same way just changing the numbers, I don't understand why it is incorrect. Any help would be appreciated.
Thank-you
A 1 mol sample of an ideal gas for which c'V = 3R/2 follows the closed thermodynamic cycle shown in Fig. 18-36. There are three legs in the cycle: an isothermal expansion, A B; an isobaric compression, B C; and a constant-volume increase in pressure, C A. The temperature during the isothermal leg is 340 K, pA = 1.8 atm, and pB = 1.2 atm. Find each of the following. (a) Va (b) Vb. (See attachment for image - ignore the pressure numbers on the graph and use the numbers given in the problem)
2. Homework Equations
Pv=nRT (initial and final)
3. The Attempt at a Solution
Pa: V= [1 mol (.08205784 L*atm/k*mol)(340K)]/1.8 atm = 15.499 L = .0155 M^3
Pb: V= [1 mol (.08205784 L*atm/k*mol)(340K)]/`1.2 atm = 23.25 L = .0232 M^3
When I plugged those answers to see if they were right, the first one came up right for the volume at A. But my response for the volume at B came up incorrect. I performed the problem in the same way just changing the numbers, I don't understand why it is incorrect. Any help would be appreciated.
Thank-you