tomMccune
- 2
- 0
Hi everyone,
I have an investigation to carry out for my Junior Certificate in Ireland. What I'm not sure about is the link between heat and temperature. I know that heat is energy, and temperature is the measurement of this energy, but I'm not sure what the link between celsius and joules is.
1. Measure the relation between the current of a circuit and the heating effect produced by that circuit2. Joule's Law - W = (I^2)(R)(t) (I'm sure this comes into it somewhere, my teacher has brought it up.
3. The way that my partner and I have carried out the experiment is:
We would measure 50ml of water and pour it into a polystyrene cup. We would then use a digital thermometer to get a reading of the temperature of the water as a control. This varied each time we got new water and measured it, probably due to weather, humidity etc. We would connect a battery to a rheostat (variable resistor), then to an ammeter, then to a wire coil, which would be submerged in the water in the polystyrene cup, then back to the other battery terminal. So, we have been waiting 8 minutes for the water to heat up, each time with a different current, then measuring the temperature of the water and the increase from the control. How would this be relavent to a measurement in joules (What formula would I use to convert my readings in celsius to Joules?)? I don't get it at all.
Here are our results (A little weird, but we can... edit them, I know they're supposed to be directly related).
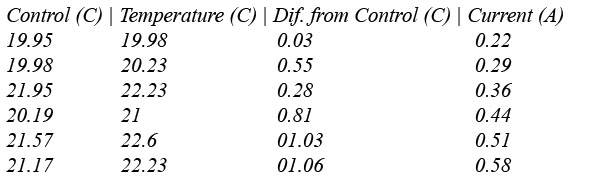
Thanks for any help, I hope I've been clear enough.
Tom.
I have an investigation to carry out for my Junior Certificate in Ireland. What I'm not sure about is the link between heat and temperature. I know that heat is energy, and temperature is the measurement of this energy, but I'm not sure what the link between celsius and joules is.
1. Measure the relation between the current of a circuit and the heating effect produced by that circuit2. Joule's Law - W = (I^2)(R)(t) (I'm sure this comes into it somewhere, my teacher has brought it up.
3. The way that my partner and I have carried out the experiment is:
We would measure 50ml of water and pour it into a polystyrene cup. We would then use a digital thermometer to get a reading of the temperature of the water as a control. This varied each time we got new water and measured it, probably due to weather, humidity etc. We would connect a battery to a rheostat (variable resistor), then to an ammeter, then to a wire coil, which would be submerged in the water in the polystyrene cup, then back to the other battery terminal. So, we have been waiting 8 minutes for the water to heat up, each time with a different current, then measuring the temperature of the water and the increase from the control. How would this be relavent to a measurement in joules (What formula would I use to convert my readings in celsius to Joules?)? I don't get it at all.
Here are our results (A little weird, but we can... edit them, I know they're supposed to be directly related).
Thanks for any help, I hope I've been clear enough.
Tom.