epsilon
- 29
- 1
I am currently trying to understand the mass attenuation coefficient (MAC) with regards to photoelectric absorption. Consider this graph:
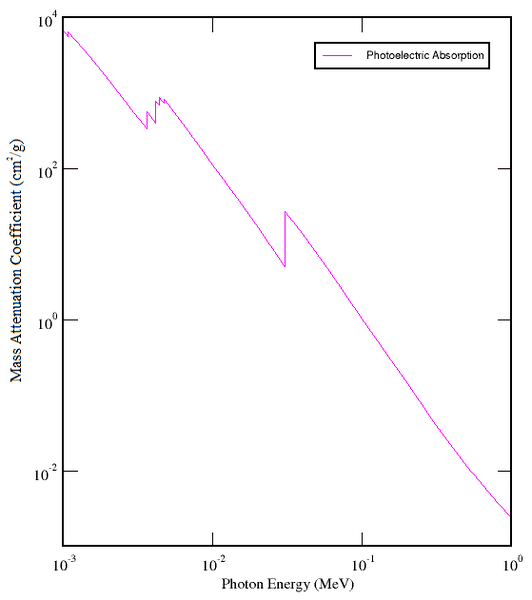
Why does this have a negative gradient? Why does the MAC decrease as the photon energy increases?
From what I understand, the MAC is the rate of energy loss of a photon as it traverses a medium, independent of the density of the medium and is therefore solely a property of the atomic arrangement, etc. Hence why would a photon be losing energy slower if it is initially higher energy? Also, I believe the jagged parts of the line are the electron excitation energies of atoms, but how does this tie into the negative gradient?
Many thanks in advance!
Why does this have a negative gradient? Why does the MAC decrease as the photon energy increases?
From what I understand, the MAC is the rate of energy loss of a photon as it traverses a medium, independent of the density of the medium and is therefore solely a property of the atomic arrangement, etc. Hence why would a photon be losing energy slower if it is initially higher energy? Also, I believe the jagged parts of the line are the electron excitation energies of atoms, but how does this tie into the negative gradient?
Many thanks in advance!