- #1
HAF
- 58
- 6
Hello,
I have a little problem. I try to find out why the second equation in the picture is correct. How can I get it? Down on the paper is what I did.
Where did the (1+V2/V1) disappear from the right side of equation?
Thank You for every help.
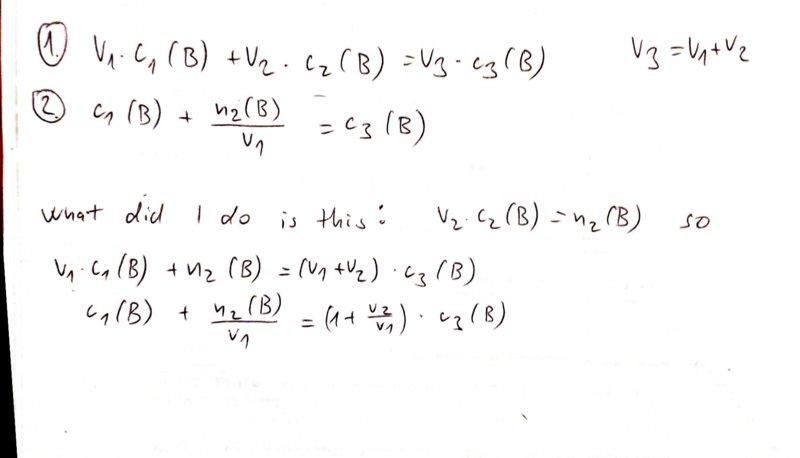
I have a little problem. I try to find out why the second equation in the picture is correct. How can I get it? Down on the paper is what I did.
Where did the (1+V2/V1) disappear from the right side of equation?
Thank You for every help.
Attachments
Last edited:
