- #1
Jeronimus
- 287
- 9
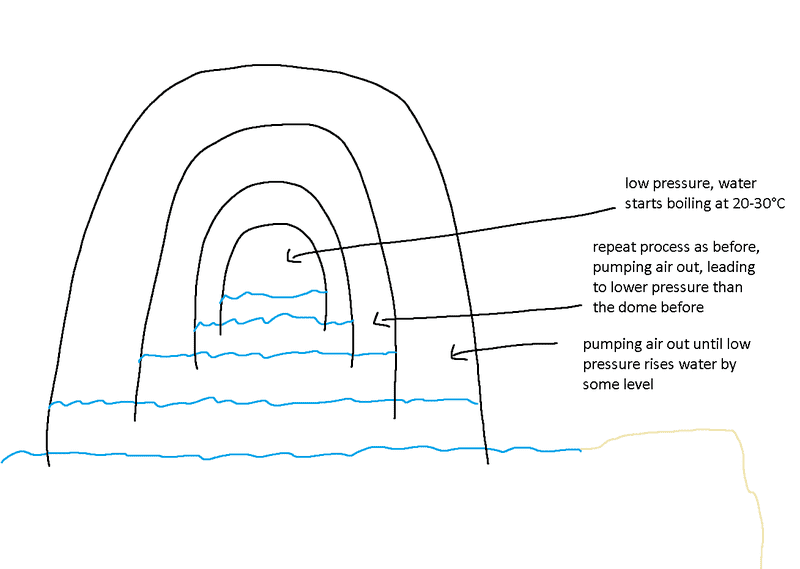
Imagine some giant glass(or other material) dome placed on top of some ocean or lake. Then pump some air out of this dome, until the water level rises a few meters under the dome.
Place another dome below the first dome, and repeat the process, resulting in a little less pressure in the second dome.
Keep repeating this, until the pressure in the final dome is low enough to get water boiling at room temperature.
Would such a device work or did my thinking go wrong somewhere?
The picture below shows how I imagined it.
Place another dome below the first dome, and repeat the process, resulting in a little less pressure in the second dome.
Keep repeating this, until the pressure in the final dome is low enough to get water boiling at room temperature.
Would such a device work or did my thinking go wrong somewhere?
The picture below shows how I imagined it.