- #1
aleferesco
- 28
- 0
The question that I have is regarding the hybridization of the Carbon atom in the molecule Carbanion
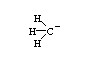
[CH3]-
My question is
Why is the carbon atom in Carbanion hybridized to sp3 orbitals instead of sp2?
shouldn't it be sp2 since the carbon atom is only making 3 bonds with hydrogen?
please explain
any help is truly appreciated
http://www.chem.uh.edu/Courses/Thummel/Chem3331/Notes/Chap4/Image178.gif
[CH3]-
My question is
Why is the carbon atom in Carbanion hybridized to sp3 orbitals instead of sp2?
shouldn't it be sp2 since the carbon atom is only making 3 bonds with hydrogen?
please explain
any help is truly appreciated
http://www.chem.uh.edu/Courses/Thummel/Chem3331/Notes/Chap4/Image178.gif
Last edited by a moderator: