- #1
Peter Forbes
- 3
- 0
I have a question about the Lagrange Multiplier method used to derive the Boltzmann distribution. I'm following the first http://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_statistics" .
I can get to this equation fine:
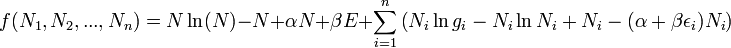
And I understand how they get the next line,
[itex]
\frac{df}{dN_i}=\ln{g_i}-\ln{N_i}-(\alpha+\beta \epsilon_i)
[/itex]
by considering N a constant when they do the partial differentiation.
However, since [itex] N=\sum_{i=1}^n N_i [/itex], can't I substitute that expression everywhere I have a N in the first equation? Then the differentiation gives me
[itex]
\frac{df}{dN_i}=\ln{(\sum_{i=1}^n N_i)}+(\alpha-1)(\sum_{i=1}^n N_i)+\ln{g_i}-\ln{N_i}-(\alpha+\beta \epsilon_i)
[/itex]
Which clearly has a different solution. Substituting for N before doing the partial derivative changes things...but why? I don't understand how one way should be any "more correct" than the other.
Thanks in advance.
I can get to this equation fine:
And I understand how they get the next line,
[itex]
\frac{df}{dN_i}=\ln{g_i}-\ln{N_i}-(\alpha+\beta \epsilon_i)
[/itex]
by considering N a constant when they do the partial differentiation.
However, since [itex] N=\sum_{i=1}^n N_i [/itex], can't I substitute that expression everywhere I have a N in the first equation? Then the differentiation gives me
[itex]
\frac{df}{dN_i}=\ln{(\sum_{i=1}^n N_i)}+(\alpha-1)(\sum_{i=1}^n N_i)+\ln{g_i}-\ln{N_i}-(\alpha+\beta \epsilon_i)
[/itex]
Which clearly has a different solution. Substituting for N before doing the partial derivative changes things...but why? I don't understand how one way should be any "more correct" than the other.
Thanks in advance.
Last edited by a moderator: