- #1
dreamspy
- 41
- 2
Here comes a pretty hard question, which not even my QM teacher has been able to answer.
When we think about one hydrogen atom, and put it in an electric field along the z-axis [tex]\bar E = \bar e_z E[/tex]. Then the potential for a hydrogen atom will look like this:
[tex]U = -\frac{e^2}{4\pi \epsilon_0 \sqrt{x^2+y^2+z^2}} - eEz[/tex]
This looks approximately like this:
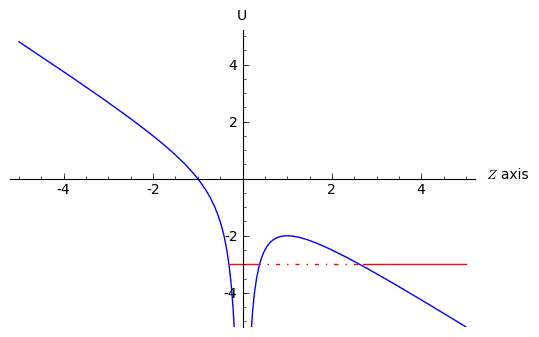
Here the blue line is the potential. Now let's say that the energy of the electron is the red line. If we let enough time go by, the electron should tunnel out of the atom and drift of into infinity in the z-direction, due to the applied electric field.
According to this then if one takes a gas of hydrogen atoms, and encloses them in a container. Then applies the electric field E, the gas should ionize and form a plasma, given enough time...
But in the lab this doesn't happen.
Why?
Regards
Dreamspy
When we think about one hydrogen atom, and put it in an electric field along the z-axis [tex]\bar E = \bar e_z E[/tex]. Then the potential for a hydrogen atom will look like this:
[tex]U = -\frac{e^2}{4\pi \epsilon_0 \sqrt{x^2+y^2+z^2}} - eEz[/tex]
This looks approximately like this:
Here the blue line is the potential. Now let's say that the energy of the electron is the red line. If we let enough time go by, the electron should tunnel out of the atom and drift of into infinity in the z-direction, due to the applied electric field.
According to this then if one takes a gas of hydrogen atoms, and encloses them in a container. Then applies the electric field E, the gas should ionize and form a plasma, given enough time...
But in the lab this doesn't happen.
Why?
Regards
Dreamspy