You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Atomic mass: Definition and 64 Discussions
The atomic mass (ma or m) is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da, or u) where 1 dalton is defined as 1⁄12 of the mass of a single carbon-12 atom, at rest. The protons and neutrons of the nucleus account for nearly all of the total mass of atoms, with the electrons and nuclear binding energy making minor contributions. Thus, the numeric value of the atomic mass when expressed in daltons has nearly the same value as the mass number. Conversion between mass in kilograms and mass in daltons can be done using the atomic mass constant
m
u
=
m
(
12
C
)
12
=
1
D
a
{\displaystyle m_{\rm {u}}={{m({\rm {^{12}C}})} \over {12}}=1\ {\rm {Da}}}
.
The formula used for conversion is:
1
D
a
=
m
u
=
M
u
N
A
=
M
(
12
C
)
12
N
A
=
1.660
539
066
60
(
50
)
×
10
−
27
k
g
,
{\displaystyle 1\ {\rm {Da}}=m_{\rm {u}}={M_{\rm {u}} \over {N_{\rm {A}}}}={M(^{12}C) \over {12\ N_{\rm {A}}}}=1.660\ 539\ 066\ 60(50)\times 10^{-27}\ \mathrm {kg} ,}
where
M
u
{\displaystyle M_{\rm {u}}}
is the molar mass constant,
N
A
{\displaystyle N_{\rm {A}}}
is the Avogadro constant, and
M
(
12
C
)
{\displaystyle M(^{12}\mathrm {C} )}
is the experimentally determined molar mass of carbon-12.The relative isotopic mass (see section below) can be obtained by dividing the atomic mass ma of an isotope by the atomic mass constant mu yielding a dimensionless value. Thus, the atomic mass of a carbon-12 atom is 12 Da by definition, but the relative isotopic mass of a carbon-12 atom is simply 12. The sum of relative isotopic masses of all atoms in a molecule is the relative molecular mass.
The atomic mass of an isotope and the relative isotopic mass refers to a certain specific isotope of an element. Because substances are usually not isotopically pure, it is convenient to use the elemental atomic mass which is the average (mean) atomic mass of an element, weighted by the abundance of the isotopes. The dimensionless (standard) atomic weight is the weighted mean relative isotopic mass of a (typical naturally-occurring) mixture of isotopes.
The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum of the masses of their constituent protons, neutrons, and electrons, due to binding energy mass loss (per E = mc2).
View More On Wikipedia.org
m
u
=
m
(
12
C
)
12
=
1
D
a
{\displaystyle m_{\rm {u}}={{m({\rm {^{12}C}})} \over {12}}=1\ {\rm {Da}}}
.
The formula used for conversion is:
1
D
a
=
m
u
=
M
u
N
A
=
M
(
12
C
)
12
N
A
=
1.660
539
066
60
(
50
)
×
10
−
27
k
g
,
{\displaystyle 1\ {\rm {Da}}=m_{\rm {u}}={M_{\rm {u}} \over {N_{\rm {A}}}}={M(^{12}C) \over {12\ N_{\rm {A}}}}=1.660\ 539\ 066\ 60(50)\times 10^{-27}\ \mathrm {kg} ,}
where
M
u
{\displaystyle M_{\rm {u}}}
is the molar mass constant,
N
A
{\displaystyle N_{\rm {A}}}
is the Avogadro constant, and
M
(
12
C
)
{\displaystyle M(^{12}\mathrm {C} )}
is the experimentally determined molar mass of carbon-12.The relative isotopic mass (see section below) can be obtained by dividing the atomic mass ma of an isotope by the atomic mass constant mu yielding a dimensionless value. Thus, the atomic mass of a carbon-12 atom is 12 Da by definition, but the relative isotopic mass of a carbon-12 atom is simply 12. The sum of relative isotopic masses of all atoms in a molecule is the relative molecular mass.
The atomic mass of an isotope and the relative isotopic mass refers to a certain specific isotope of an element. Because substances are usually not isotopically pure, it is convenient to use the elemental atomic mass which is the average (mean) atomic mass of an element, weighted by the abundance of the isotopes. The dimensionless (standard) atomic weight is the weighted mean relative isotopic mass of a (typical naturally-occurring) mixture of isotopes.
The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum of the masses of their constituent protons, neutrons, and electrons, due to binding energy mass loss (per E = mc2).
View More On Wikipedia.org
-

What is the definition of the dalton (Da) unit?
I am very confused with this question, firstly, I am unsure what is meant by the "unified atomic mass unit" I know that it is defined as "1/12 of the mass of an atom of carbon 12", but this sounds like it takes into account the electrons, i.e that is this means to me that unified atomic mass...- heroslayer99
- Thread
-
- Tags
- Atomic mass Proton
- Replies: 10
- Forum: Introductory Physics Homework Help
-
A
B Understanding Mass Excess and Atomic Mass in Nuclear Physics
I see this term comes up in KAERI table and elsewhere. Now let me guess here and tell me whether I'm correct. The only element whose atomic mass expressed in AMU is exactly a round number is C12, for all other elements it's either bit more or less so in order not to have to write long numbers in...- artis
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 7
- Forum: High Energy, Nuclear, Particle Physics
-
T
B Reduced mass to atomic mass units conversion help please
Hello I am trying to understand how to write the reduced mass into atomic mass units but i am confused how it was done. The equation is $$m_1m_2/(m_1+m_2)$$ For two similar masses in my particular case i have: $$m^2 / 2m = 1/2 * m$$ Then to convert to atomic mass units, the book says it...- TheCelt
- Thread
- Replies: 3
- Forum: Atomic and Condensed Matter
-

Gram atomic mass -- what exactly is it?
It is stated that the gram atomic mass of an element is its atomic mass represented in grams. The gram atomic mass of sodium is said to be 23g (isn't this number way to big to represent the mass of an atom?!) but if its the mass of one mole of sodium it makes sense(but won't that be molar mass)...- Hamiltonian
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 6
- Forum: Chemistry
-

Why wasn't fluorine used as a standard for atomic mass?
Hi, I found the two sections in red a little confusing. I'd appreciate it if you could help me with it. Source: https://en.wikipedia.org/wiki/Atomic_mass#History The existence of isotopes was first suggested around 1913[reference]. The first periodic table by Mendeleev in 1869 used...- PainterGuy
- Thread
-
- Tags
- Atomic Atomic mass Isotopes Mass
- Replies: 6
- Forum: Chemistry
-
A
B Factors contributing to the atomic mass of an atom
I was studying about atomic masses and realized that even if we say that the atomic mass unit corresponds to 1/12 of the mass of a carbon atom. why is it that even particular isotopes of elements have atomic mass in decimal values. 1/12 of a carbon atoms mass should equal to the mass of a...- Avalon_18
- Thread
-
- Tags
- Atom Atomic Atomic mass Factors Mass
- Replies: 3
- Forum: Atomic and Condensed Matter
-

Is AMU the unit for both 'atomic mass' and 'molecular mass'?
Is 'AMU' the unit for both 'atomic mass' and 'molecular mass'?- HCverma
- Thread
-
- Tags
- Atomic mass Mass Unit
- Replies: 2
- Forum: Chemistry
-
K
Charges of ions in a mass spectrometer
Homework Statement In a mass spectrometer, a few 126C ions are deflected with a radius of 12.9 cm. The ions have a speed of 5.67*10^4 m/s when they enter the field. The magnetic field has a strength of 3.2*10^-2 T. The charge of these ions must be ... SOLUTION: We use the formula: i F=qvB =...- Karagoz
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Convert atomic mass into Joules
Homework Statement How much energy is created when 0,0188828901 atomic mass is converted into energy (Joule) Homework Equations E = m * c^2 speed of light = 299792458 1 atomic mass unit = 1.660539040e-27 kg 1 atomic mass unit = 931,494028 MeV 1 eV = 1,6021766208e-19 Joule The Attempt at a...- Sebastiaan
- Thread
-
- Tags
- Atomic Atomic mass Convert Joules Mass
- Replies: 8
- Forum: Introductory Physics Homework Help
-

I Regarding the precision and accuracy of Atomic mass
The website of CIAAW list the 2012 atomic mass in Daltons (Atomic Mass Units) and mention the atomic mass of stable isotopes followed by an additional number between braces. For example Helium-4: 4.002 603 2541(4) My first question is what does the number between braces mean? I assume it is some...- Sebastiaan
- Thread
- Replies: 2
- Forum: Atomic and Condensed Matter
-
Atomic Mass of Element X: Solving with John Dalton Ratios
I believe that this question may be solved by the John Dalton ratios, but, I'm confused: While traveling to a distant universe, you discover the hypothetical element “X.”You obtain a representative sample of the element and discover that it is made up of two isotopes,X-23 and X-25. To help your...- dshipp17
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 13
- Forum: Biology and Chemistry Homework Help
-
N
B Convert atomic mass to metric
I have a complete brain freeze sorry, and cannot work this out. I have a helium atom of 4.0 amu and there are 6.0 x 10^24 atoms. How do I calculate the total mass in kg please? Thanks- Nick Jarvis
- Thread
-
- Tags
- Atomic Atomic mass Convert Mass Metric
- Replies: 2
- Forum: Atomic and Condensed Matter
-
J
Calculating the weight of 1 atomic mass unit
I'm trying to deduce the weight of 1 atomic mass unit (##1u##) in ##kilograms## from the following scenario: One atomic mass unit is 1/12 of the weight of a ##^{12}C## atom in its ground state. A ##^{12}C## atom consists of 6 protons, 6 neutrons and 6 electrons. This means that $$1u =... -
D
A Change in average atomic mass in universe through time?
My understanding is that matter tends to converge and form stars and in those stars fusion creates heavier atoms from lighter atoms, and this process repeats continuously. if this is true wouldn't it imply that throughout these star cycles the average atomic mass will just increase, and as the...- dkm0038
- Thread
- Replies: 6
- Forum: Astronomy and Astrophysics
-

Isotopes, atomic mass, abundance
Homework Statement Suppose the element A contains three isotopes, 86A (atomic mass 85.909 u, abundance 16.19%) 87A (atomic mass 86.908, abundance 7.00%) and 88A (atomic mass 87.906 u). What would be the atomic mass of A that would appear in the periodic table? Homework Equations (A1 x %A1) +...- CMATT
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
F
Atomic Mass and Rest Energy
Homework Statement "You might wonder how six protons and six neutrons, each having a mass larger than 1 u, can be combined with six electrons to form a carbon-12 atom having a mass of exactly 12 u. The bound system of carbon-12 has a lower rest energy than that of six separate protons and six...- fridakahlo
- Thread
-
- Tags
- Atomic Atomic mass Energy Mass Rest
- Replies: 3
- Forum: Introductory Physics Homework Help
-
N
I Uncertainty about relative atomic mass
For the uncertainty about relative atomic mass. Such as for H-1 there is 1.00794(7)u. what exactly does the "7"means? Is it 1.007947? or 1.00794(+/-)0.000007? or something else? ref: http://www.ciaaw.org/pubs/TSAW-2007.pdf- Neutroniclad
- Thread
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
B
What is the difference between amu and u in atomic mass units?
Homework Statement What is the difference between amu and u? In my book they seem to use u but on my online homework it uses u and it seems to be the same thing. Homework Equations chemistry has too many special units The Attempt at a Solution atomic mass unit 1/12 mass of an atom of carbon...- brycenrg
- Thread
-
- Tags
- Atomic Atomic mass Mass Units
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

WTA : does atomic mass matter to atom's radius?
Homework Statement so my friend asked this question: "what's the atomic radius ratio between He-3 and He-4?" 2. Homework Equations all i know is this equation : r =nh/mv 3. The Attempt at a Solution i thought the ratio will be same because both He-3 and He-4 have only two electrons. Correct...- adi adi
- Thread
-
- Tags
- Atomic Atomic mass Mass Matter Radius
- Replies: 3
- Forum: Introductory Physics Homework Help
-

What is the Atomic Mass of Deuterium According to Parthey's Measurements?
In the same article which describes the accurate measurement of the Hydrogen 1s-2s transition at: 2 466 061 413 187 035 (10) Hz, CG Parthey also measures the frequency difference between Hydrogen and Deuterium which he gives as: 670 994 334 606(15) Hz. Assuming that the transition...- neilparker62
- Thread
-
- Tags
- Atomic Atomic mass Deuterium Mass
- Replies: 5
- Forum: Atomic and Condensed Matter
-

Precision measurements of atomic mass.
Hello I have been trying to find out how the data for the atomic mass of different isotopes is processed. I am vaguely familiar with mass spectrometry and other techniques used for the measurements and I know that the experimental results are coordinated by the Atomic Mass Data Centre. I am...- Dadface
- Thread
- Replies: 5
- Forum: Atomic and Condensed Matter
-
J
Why is the relative atomic mass always changing?
And why the range of changing is different when we use different atoms as standards?- Johannah Wu
- Thread
-
- Tags
- Atomic Atomic mass Mass Relative
- Replies: 1
- Forum: Chemistry
-
J
Why hydrogen is not used to compare the relative atomic mass
Is that because of some historical reasons or E=mc2(i mean something about binding energy)?- Johannah Wu
- Thread
- Replies: 1
- Forum: Chemistry
-
O
Finding Atomic Mass Unit by Subatomic Particles
If one atomic mass unit is 1/12 of a C-12 atom, so 1 amu must be; 1 Amu = 1/2 ( Mass of proton + Mass of neutron + Mass of electron ) But the equation gives the answers that 1 amu is 1,6737 10^(-27) kg, which is different than the internet says I am using very sensitive values for the... -
S
Understanding atomic mass units (amu) and weight of particles
I read that a C12 atom has an atomic mass unit of 12 amu's. A C12 atom has 6 protons, 6 neutrons, and 6 electrons. If the mass of a proton is 1.00728 amu, the mass of a neutron is 1.00867 amu, and the mass of an electron is .000549, how can it's total amu be 12...- solarcopper
- Thread
- Replies: 2
- Forum: Chemistry
-
A
Calculating average atomic mass
The relative abundance of various isotopes of silicon is as Si(28) = 92.25%, Si(29) = 4.65% and Si(30) = 3.10 % . Calculate the average atomic mass of Silicon. Ans : 28.11 u Attempt. : I don't know how to start this problem . -
M
Finding the unknown atomic mass of element X
Homework Statement Suppose a nameless element (X) was found to occur in three different substances (A, B and C). The relative molecular masses of A, B, and C were found to be 25.80, 43.70 and 83.50 respectively, compared to an assigned value of exactly 2.00 for H2. Subsequently, these...- maiad
- Thread
-
- Tags
- Atomic Atomic mass Element Mass
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
Z
Simple Atomic Mass Unit question
Homework Statement The uranium isotope 235U captures a neutron and undergoes fission to produce 93Rb and 141Cs. Calculate the energy released in this process. The nuclear masses of the relevant isotopes are 235U 235.0439u, 93Rb 92.9217u, 141Cs 140.9195u Homework Equations...- ZedCar
- Thread
-
- Tags
- Atomic Atomic mass Mass Unit
- Replies: 6
- Forum: Introductory Physics Homework Help
-
N
Whether sum of weight of proton and neutron is equal to atomic mass?
Dear friend, I am getting confused in this question;what i learned till now is that number of proton+number of neutron=atomic mass. But while searching the net i got this equation atomic mass = mass a x fract a + mass b x fract b. can anyone tell me which is the correct (not approximate)... -
R
Doubts regarding Atomic mass,relative atomic mass and mass number.
I was reading my science textbook and they have introduced the concepts of atomic mass,relative atomic mass and mass number.Here are some doubts I have- 1)Is atomic mass a ratio ? 2)1/12th of a C-12 atom is half a proton,half a neutron and half an electron.Since mass of an electron is... -
P
What is the difference between atomic mass and molar mass in chemistry?
I have a quiz on Tuesday at chemistry, and I began reviewing for it. The subject is the Ideal Gas Law. I pretty much got the hang of it, except that I have one tiny problem. I'm kind of rusty on chemistry, and that's preventing me from getting a good grip. I've looked on Wikipedia, but it seems...- psyhprog
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 1
- Forum: Chemistry
-
V
Atomic mass units(old and new)
Homework Statement Prior to 1961, the physical standard for atomic masses was 1/16 the mass of the oxygen atom. The new standard is 1/12 the mass of the carbon atom. Determine the conversion factor needed to convert from old to new atomic mass units. How did this change affect the value of the...- Visceral
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 11
- Forum: Biology and Chemistry Homework Help
-
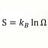
1 gram H / 1 atomic mass unit (grams ) What is wrong with the math here ?
One atomic mass unit = .001660565 x 10-24 grams One gram Hydrogen / .001660565 x10-24 grams = 6.02 x 10 26 ? This should be Avogadros number 6.02 x 1023 what is the mistake here ? -
A
Find the difference in atomic mass between the two isotopes
Homework Statement Two isotopes of a certain element have binding energies that differ by 5.03 MeV. The isotope with the larger binding energy contains one more neutron than the other isotope. Find the difference in atomic mass between the two isotopes.Homework Equations 1u=931.5MeVThe...- Ailiniel
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
I
Finding Atomic Mass: How Scientists Like Mendeleev Determined it
I have always been told that scientists such as Mendeleev used atomic mass to list the known elements of their time. I have always been curious as to how those early scientists actually determined the atomic masses of those known elements. What kind of experiment or process/principle was used...- Infrasound
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 1
- Forum: Atomic and Condensed Matter
-
S
Correct definition of Relative atomic mass
Homework Statement Explain what you understand by the term Relative Atomic Mass The question is equal to three marks, though i don't think I've put enough for all the marks: The Attempt at a Solution The mass of an atom relative to one atom of carbon-12. I would think that's only...- synkk
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
Y
Relative Atomic Mass: Definition & Explanation
Can somebody please explain for me ?? -
A
Finding the atomic mass of a hypothetical element.
Here is a problem I have. Not really sure where to start. I'm not looking for the answer, I just want someone to point me in the right direction. A hypothetical element X has three isotopes: 40X, 41X, and 42X. Their abundances are 72.00%, 9.00% and 19.0% respectively. What is the atomic mass of...- Aeropsia
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
F
Atomic Mass in a Mass Spectrometer
Homework Statement Natural carbon consists of two different isotopes. The isotopes have different masses, which is due to different numbers of neutrons in the nucleus; however, the number of protons is the same, and subsequently, the chemical properties are the same. The most abundant isotope...- futurepocket
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
M
Why does Atomic Mass Differ from # of Protons & Neutrons?
Why atomic mass doesn't match the number of protons and neutrons in it? for example mercury mass is 200 gmol but it has only 80 protons and 80 neutrons. From where does 40 gmol come from? -
T
Calculating Approximate Atomic Mass of Lead | Isotopes of Pb and Percent by Mass
Homework Statement 1. The Table below lists the isotopes of Pb and their percent by mass: Isotope % Mass 82p, 122n 1.37% 82p, 124n 26.25% 82p, 125n 20.82% 82p, 126n 51.55%(a) Using these data, calculate the approximate atomic mass...- tigerwoods99
- Thread
-
- Tags
- Approximate Atomic Atomic mass Mass
- Replies: 29
- Forum: Biology and Chemistry Homework Help
-
S
Atomic mass unit and Molar mass
Hi My textbook states that say 50 amu/atom = 50 g/mol. Which does not really make any sense. I am not sure how to get the atom unit. Since on wikipedia it says u = Molar-mass/Na Molar-mass(g/mol) = u*Na I also know that 1gram/Na = 1u- SpartanG345
- Thread
-
- Tags
- Atomic Atomic mass Mass Unit
- Replies: 10
- Forum: Chemistry
-
N
What is the method for calculating atomic mass from isotopes?
hi i was wondering if anybody could help me? for calculating if there's a missing part how do i solve it ? i have a picture ... how would i figure out the third part ..?- neon1234
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 2
- Forum: Chemistry
-
K
Atomic Mass of Contaminated Iodine
Homework Statement Naturally occurring iodine has an atomic mass of 126.9045. A 12.3849-g sample of iodine is accidentally contaminated with 1.00070 g of ,129I a synthetic radioisotope of iodine used in the treatment of certain diseases of the thyroid gland. The mass of129I is 128.9050 ...- Ki-nana18
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
R
Nuclear physics: neutral atomic mass vs. atomic mass
Homework Statement I am preparing a presentation on nuclear reactor technology. To demonstrate the mass-energy equivalence, I am trying to calculate the binding energy of some heavier isotopes. The problem is that, when I substitute the values that I have into the equation, I get a...- rhombus
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
C
Chemistry - isotpic mass, atomic mass, mass number
Homework Statement The two isotopes of antimony have masses 120.90 and 122.90, with a relative abundance of 57.25% and 42.75% respectively. Using these figures, distinguish clearly between atomic mass, isotopic mass, and mass number. Homework Equations The Attempt at a Solution...- clairez93
- Thread
-
- Tags
- Atomic Atomic mass Chemistry Mass
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
M
How to Calculate the atomic mass of this isotope?
Q: The actual mass of one atom of an unknown isotope is 2.18 x 10^-22g. Calculate the atomic mass of this isotope. `How do I calculate it? help =]- miiizpiiink18
- Thread
-
- Tags
- Atomic Atomic mass Isotope Mass
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
T
How to Calculate the Atomic Mass of an Unknown Element M in MCl?
unknown ellement called M reacts with Cl to make MCl 0.7718 gram of MCl reacts with AgNO_3 as a result we get 2.6091 gram of AgCl. what is the atomic mass of M ??- transgalactic
- Thread
-
- Tags
- Atomic Atomic mass Mass
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
P
Atomic Mass of Rare Carbon Isotope: Solving the Puzzle
Homework Statement Natural carbon consists of two different isotopes (excluding 14C, which is present in only trace amounts). The isotopes have different masses, which is due to different numbers of neutrons in the nucleus; however, the number of protons in the same, and subsequently the...- phy112
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-
G
Chemistry The Atomic Mass of M
Homework Statement When M2S3 is heated in air, it is converted to MO2. A 4.000g sample of M2S3 shows a decrease in mass of .277 g when it is heated in air. What is the average atomic mass of M? Homework Equations The Attempt at a Solution I don't not really know how to tackle this...- George3
- Thread
-
- Tags
- Atomic Atomic mass Chemistry Mass
- Replies: 2
- Forum: Biology and Chemistry Homework Help