You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Internal: Definition and 991 Discussions
An internality is the long-term benefit or cost to an individual that they do not consider when making the decision to consume a good or service. One way this is related to behavioral economics is by means of the concept of hyperbolic discounting, in which immediate consequences of a decision are disproportionately weighed compared to the future consequences. A potential cause is lack of access to full information regarding the associated costs and benefits prior to consumption. This contrasts with traditional economic theory, which makes the assumption that individuals are rational decision makers who take all personal costs into account when paying for goods and services.One example of a positive internality is the long run effect of exercising, if these are not taken into account when deciding whether to exercise. Future benefits that an individual may not take into consideration include a diminished risk of heart disease and higher bone density. A common example of a potential negative internality is the effect of smoking cigarettes on those who smoke. For the effect of secondhand smoke, see externality. Statistically, 80% of smokers want to quit, and 54% of people who are serious about quitting fail in a week or less. This implies that they do not act in their long-term best interest due to short-term discomfort, also known as a self-control problem. If the demand for cigarettes has a high price elasticity of demand, which evidence seems to suggest, the government can combat the negative internality by raising taxes. It is important to note that elasticity might change based on location and knowledge about the harmful health effects of smoking. In traditional economic theory, a tax diminishes the welfare of the poor because the tax burden shifts to low-income communities, as fewer can afford the good (cigarettes), and horizontal equity (economics) is distorted. However, behavioral economic theory suggests that the tax is not regressive if low-income communities have higher (healthcare) costs and more price sensitivity than individuals with higher incomes. Taxes imposed to combat internalities are most effective when they target a specific good. A tax on junk food could apply to a large variety of goods that are widely consumed, and the cost of the tax might be perceived as more detrimental than beneficial for society. A major issue with creating effective legislature against negative internalities is that the tax imposed should only reflect the cost that individuals do not factor into their consumption decisions. The difficulty in measuring individual knowledge is an obstacle to developing new policies. Another point of concern is that the group benefitting from the tax, such as smokers who want to quit, must be sizable enough to offset any backlash from tobacco companies and lobbyists.
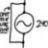
In the following graphs, D' and S' are the demand and supply curves if producers and consumers take all external costs (EC) into consideration. The tax attempting to prevent the internality should be set equal to the difference between D and D' at the optimal quantity, which is the unmeasured internal cost (IC).
View More On Wikipedia.org
In the following graphs, D' and S' are the demand and supply curves if producers and consumers take all external costs (EC) into consideration. The tax attempting to prevent the internality should be set equal to the difference between D and D' at the optimal quantity, which is the unmeasured internal cost (IC).
View More On Wikipedia.org
-

Does the Carnot heat engine law apply to an internal combustion engine?
The law says that the maximum thermal efficiency of a heat engine can be 88%. If I have and internal combustion engine using 9.8 cc/min instead of 25 to 30 cc/min and the other accepted figure is a 2L 4 cylinder engine for idle is it uses approx 5 HP of fuel just just to idle. So I am doing...- smokingwheels
- Thread
- Replies: 21
- Forum: Thermodynamics
-
What is the Minimum Refractive Index for Total Internal Reflection?
Homework Statement See figure attached for problem statement. Homework Equations The Attempt at a Solution Using Snell's Law, n_{1}sin(\theta_{1}) = n_{2}sin(\theta_{2}) n_{1} = \frac{n_{2}sin(\theta_{2})}{sin(\theta_{1})} Where, \theta_{1} = 30^{o}, \theta_{2} =...- jegues
- Thread
- Replies: 5
- Forum: Precalculus Mathematics Homework Help
-
P
Change in internal energy when ice melts to water at 0C
If we melt ice at 0'C to water at 0C, what is the change in the internal energy of the ice-water system? As per the first law of thermodynamics, ΔU = ΔQ +ΔW where they are increase in internal energy, heat flow to the system and work done on the system respectively. If we melt ice at 0C, we...- Pranav Jha
- Thread
- Replies: 18
- Forum: Mechanics
-
S
Electric field in a conductor with an internal cavity
I'm not entirely sure if this is meant to be in the "advanced physics" section, it's for my undergraduate degree anyway. Homework Statement A conducting sphere of radius R, carries a total charge of q. It is surrounded by an electrically neutral conducting shell with an inner radius, a, and...- Silversonic
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
R
Heat conduction through a slab with internal heat generation
Homework Statement There's a slab of a material with temperature T1 on the left and the T2 on the right. The thickness of the material is l with area A. In the centre, there is heat generation Qvol in the centre, which is a thin rod. Find the heat transfer Q through the material. Homework...- rafehi
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-
F
What is the driving force to increasing the internal resistance of a battery
What is the "driving force" to increasing the internal resistance of a battery Homework Statement A brand new battery almost have no resistance, but overtime when the internal resistance is great enough, the battery "dies". So my question is, what is causing the internal resistance to...- flyingpig
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
T
Help Internal Energy of a Liquid
In thermodynamics Change in Internal Energy = Heat - Work (\DeltaU=Q-W) During vaporization my Heat is mass*Latent Heat (Q=mL) and Work is Pressure*change in volume (W=P\DeltaV), so \DeltaU=mL-P\DeltaV. Let's take any of the natural monoatomic gases, Neon in this case. \Delta = Volume...- tamiller07
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
B
Find emf and internal resistance of a battery
Homework Statement When a 60 \Omega resistor and a 120 \Omega are connected in parallel across a battery there is a current of 700 mA flowing through the battery. When the 120 Ohms resistor is disconnected from the circuit the current through the battery drops to 500 mA. What is the emf and the...- Blu3eyes
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
U
Internal resistance, dual power source problem
Homework Statement An emergency supply consists of a small diesel engine driving an alternator. The output of the alternator is rectified and smoothed to produce DC. This has has to supply 10 amps of 110V emergency lighting and charge a 110V battery of internal resistance 2 ohms, If the...- ukdave
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
B
Internal magnetic field experienced by H atom Electron
Homework Statement A 21 cm spectral line corresponds to the flipping of the electron in a hydrogen atom from having its spin parallel to the spin of the proton to having it anti-parallel. Find the internal magnetic field experienced by the electron in the hydrogen atom. Homework Equations...- Bosley
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
S
Internal energy and thermodynamic entropy
As a practical matter, the internal energy of a system is treated as a state function of a the system and is concerned only with the kinetic energy of particles, not the potential energy. So for thermodynamic entropy, S=E/T, (S=entropy, T= absolute temperature) I'm considering E to be kinetic...- SW VandeCarr
- Thread
- Replies: 7
- Forum: Thermodynamics
-
K
Total internal reflection zero
How is that in refraction of light energy is lost but not in case of total internal reflection? Is the loss in total internal reflection exactly zero? -
T
Groups - Internal Direct Product
Homework Statement [PLAIN]http://img689.imageshack.us/img689/3047/directproduct.png < denotes a subgroup. \triangleleft denotes a normal subgroup. The Attempt at a Solution Have I done (a) correctly? 0 \in A so A \neq \emptyset If a=x+ix and b=y+iy then ab^{-1} = x-y + ix...- Ted123
- Thread
- Replies: 2
- Forum: Calculus and Beyond Homework Help
-
D
Internal Pressure of a Star
Homework Statement Assume a star is in hydrostatic equilibrium and that the density of the star is follows \rho \propto \frac{1}{r^{a}} where \r is the distance from the centre of the star and \r is a constant. Derive an relation for the pressure of the star as a function of \r...- dats13
- Thread
- Replies: 3
- Forum: Advanced Physics Homework Help
-
C
Heat transfer internal flow through circular tube.
I have an enclosure in which the electrical components put off 10,500BTU/hr. At the top of the enclosure the internal hot air is in contact with circular aluminum tubes. Ambient outside air at 70 degrees F is blown through the center of the tubes at 500cfm. Internal hot air and outside ambient...- CruiserFJ62
- Thread
- Replies: 2
- Forum: General Engineering
-
D
Internal Resistance of a Battery problem
Homework Statement A voltmeter whose resistance is 1000 ohms measures the voltage of a worn out 1.5V battery as .9V. What is the internal Resistance Homework Equations The Attempt at a Solution In order to do this, I Solved for the Equiv Resistance of the circuit (leaving in...- dancergirlie
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
M
Internal Direct Sum of Commutative Rings: Is I + R/I = R?
For a commutative ring R and an ideal I, is it true that I \oplus R/I \cong R ? I know in some cases this is true, and I know it's true for finitely-generated Abelian groups, but is it true for any commutative ring? In other words, we know that R/I is isomorphic to some ideal in R, call this...- markiv
- Thread
-
- Tags
- Direct sum Internal Sum
- Replies: 6
- Forum: Linear and Abstract Algebra
-
A
Struggling with Total Internal Reflection? Need Help for Exam Prep?
Homework Statement Hi I wonder if anyone can help. I am having difficulty drawing refracted and total internally reflected rays. I have an exam on Monday. Can anybody recommend good websites that can help? Slight panic. Thanks Homework Equations The Attempt at a Solution- aurao2003
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
N
Thermodynamics question; internal energy and heat?
Homework Statement A helium balloon with a volume of 1m^3 and temperature of 27 deg c is placed in liquid nitrogen in an open container and cools to a temperature of 77 K. Assume that helium behaves like an ideal gas at constant atmospheric pressure and find the new volume of the gas in the...- name_ask17
- Thread
- Replies: 15
- Forum: Introductory Physics Homework Help
-
E
Why Does a Current Source Have Internal Resistance in Parallel?
hello.. this is my first post..forgive me if my question is wrong.. Why does a current source is modeled in such a way that it has its internal resistance in parallel ? Why can't it have its internal resistance in series ? The question may seem awkward , but please answer me.. thanks in advance..- electroboy12
- Thread
- Replies: 3
- Forum: Electrical Engineering
-
D
What is the Internal Energy of an Ideal Gas at Given Conditions?
Homework Statement A container of volume 0.72 m^3 contains 1.4 mol of argon gas at 24 degrees C. Assuming argon behaves as an ideal gas, find the total internal energy of this gas. The value of gas constant is 8.31451 J/mol x K. Answer in units of J. Homework Equations PV=nRT...- dlthompson81
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
M
Total Internal reflection is Glass Prism Question
Question about Total Internal Reflection? My textbook says that for total internal reflection to occur 2 conditions need to be met. Light is traveling more slowly in the first medium than the second medium and no.2 is the angle of incidence must be large enough for no refraction to occur. My... -
M
Total Internal Reflection in Glass prism Question?
Question about Total Internal Reflection? My textbook says that for total internal reflection to occur 2 conditions need to be met. Light is traveling more slowly in the first medium than the second medium and no.2 is the angle of incidence must be large enough for no refraction to occur. My...- Mohd95
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
P
Calculating Internal Energy Absorbed in Truck Cab After Collision
How to calculate Energy absobed by the components of a Truck Cab after crash test?? Hi, I have a question regarding the calculation of internal energy of components after collision. In conducting the frontal impact test as per the ECE R29 regulation, a swing bob has to hit the front of...- prasadpatrudu
- Thread
- Replies: 4
- Forum: Mechanical Engineering
-
J
Does different loads produced different internal resistance ?
hi, if suppose a battery is 100% charged and is capable of delivering 12V. Now if i connect a bulb of 40watts across it then their will be a voltage drop and the internal resistance can be calculated. If the same battery with 100% charge is used with a 80watts bulb then again their will be a...- jak9
- Thread
- Replies: 4
- Forum: Electromagnetism
-
A
Thermodynamics: Internal Energy of a Gas
Homework Statement Helium (He), a monatomic gas, fills a 0.010-m^3 container. The pressure of the gas is 6.2 multiplied by 10^5 Pa. How long would a 0.38-hp engine have to run (1 hp = 746 W) to produce an amount of energy equal to the internal energy of this gas? Homework Equations...- Azndoode1
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
S
Is Internal Energy of Ideal Gas Really Only Dependent on Temperature?
We known U=3/2nRT (monatomic ideal gas), just depends on temperature. Most texts assert connecting U and Q with constant volume condition and say"\Delta U = nCv\Delta T for any process because of internal energy only depends on temp". I think that statement is very strange. Deriving...- s943035
- Thread
- Replies: 7
- Forum: Classical Physics
-
R
Internal energy after evaporation?
Homework Statement Two grams of water are sealed in a rigid con- tainer; then the water is vaporized by heating. 1664 cal of heat is needed for vaporization. What is the change in internal energy of the water? Answer in units of J. Homework Equations 1 kcal= 4,186 J The Attempt...- Rblswimmer456
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Internal Combustion Engine Loading
Hey people I intend to self learn IC engines. I am a a little confused by what this sentence says in my textbook. "An idling engine is one which operates at no load and with nearly closed throttle" I basically want to know what loading means in this particular context? And what else can...- Sohan
- Thread
-
- Tags
- Combustion Engine Internal
- Replies: 9
- Forum: Mechanical Engineering
-
A
Internal resistance of a battery
Ok guys I need help! Its been a while since I last opened a physics workbook and now I'm back to studying to try and further my career and I've come across an internal resistance question that I can't get my head around! A resistance of 10.5 ohms is connected across the terminals of a battery...- AvoHead
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
Internal Energy: Evaporation & 1st Law of Thermodynamics
hi, Can anybody explain the phenomenon behind internal energy? and what is the change in internal energy when water evaporates. Please do give reference to first law of thermodynamics. Thanks in advance.- chocofingers
- Thread
- Replies: 4
- Forum: Classical Physics
-
J
Finding Internal Forces With Joints & Sections
I have the truss shown, I'm supposed to find the internal force for each of the vertical members...I understand method of joints and method of sections, but can you use both method of joints and sections in the same problem...for example I found Ly=52.5 and Ry=32.5 using moments and summing in...- jhox08
- Thread
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
B
Thermal Physics - Internal Energy
Homework Statement A gas obeys the equation p(V-b) = RT and has cv independent of temperature. Show: (a) the internal energy is a function of temperature only (b) the ratio cp/cv is independent of temperature and pressure (c) the equation of an adiabatic change has the form p(V-b)^gamma...- bon
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Total internal reflection mirror?
Hi all, From what I understand, total internal reflection reflect 100% of light. Suppose you had a transparent material with a critical angle of 45 degrees. Now say you had a cone with a 45 degree angle, and you shined monochromatic light in through the base. Would 100% be reflected (minus...- Smacal1072
- Thread
- Replies: 1
- Forum: Optics
-
M
Internal field in a long narrow rod
Homework Statement A long narrow solid rod has an atomic density 5 \times 10^{28}m^{-3} each atom has a polarizability of 10^{-40} . Find the internal field when an axial field of 1 V/m is applied.Homework Equations im not sure what formula to use but here's one: p = E_{loc} \sum_{r} n_j...- Mechdude
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
J
Truss - find internal force in each member
Homework Statement please see the image, I am supposed to find the internal force in each member of the truss and state if its in compression or tension Homework Equations The Attempt at a Solution here is what I have so far (all in kN)... My=40 up Gy=80 up Mx=0 from joint G... -
J
Internal force, members of truss
please see the attached image, I'm supposed to find the internal force in each member of the truss. I used moments to find My=40 and Gy=120, I then moved on to FBD of joint G where I found Fgh=30kN in tension, and Fga=50kN in compression, next I did joint A to find Fab=30kN in tension... -
P
How is kinetic energy and momentum conserved in an internal explosion?
Homework Statement an internal explosion breaks an object, initially at rest, into two pieces, one of which has 1.5 times the mass of the other. If 7500 J were released in the explosion, how much kinetic energy did each piece acquire. Homework Equations K1+K2=7500J...- pb23me
- Thread
- Replies: 14
- Forum: Introductory Physics Homework Help
-
P
Internal explosion, kinetic energy prob
Homework Statement an internal explosion breaks an object, initially at rest, into two pieces, one of which has 1.5 times the mass of the other. If 7500 J were released in the explosion, how much kinetic energy did each piece acquire. Homework Equations mgyi+1/2mvi2=mgyf+1/2mvf2 The...- pb23me
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
K
Julie's Jump: Solving Kinetic Energy & Internal Energy Problems
Homework Statement Julie has a mass of 60 kg. In an experiment she crouched down, then jumped straight up. Her lab partners measured the height of her center of mass above the floor at three instants: 1) 0.43 m when crouched down; 2) 1.02 m just as her feet were leaving the floor; 3) the...- Kreamer
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Take a sector of this circle with internal angle
Problem: Given a circle of radius 1. Take a sector of this circle with internal angle A, where 0=<A=<pi/2. Find a formula for the radius of the smallest circle that will perfectly fit this sector, as a function of A. Solution. I used laws of sine and cosine and came up with...- sutupidmath
- Thread
- Replies: 15
- Forum: General Math
-
K
Show that the Internal Energy of an Ideal gas is a Function of T only
Homework Statement Show that internal energy U = U(T) only for an ideal gas who'se equation of state is: P(V-b) = RT (the claussius equation for n moles of gas) Homework Equations Thermodynamic Equation of state: \left(\frac{\partial U}{\partial V}\right)_T =...- knowlewj01
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
K
Thermodynamic Potentials - Internal energy problem.
Homework Statement For Cyclohexane Boiling point T_b = 80.1°C P = 1atm \Delta H_{vap} = 30.1 kJ/mol Determine at phase change liquid to vapour, Changes in: a)Entropy per mole b)Gibbs free energy per mole c)Internal energy per mole assume ideal gas behaviour for the vapour...- knowlewj01
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
P
Torque, Internal forces & external forces.
Hi, I had a question about Torque, Internal forces & external forces. Here are 2 examples helping me to state my question: 1) The Earth is rounding the sun with respect to the Sun. The forces which are acting on the system are 2 gravitational forces that are action & re-action. The Force... -
A
Micro and macro internal energy approach
Why is internal energy not sufficient to compare states? Thank you for your answer.- aviatorx
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
R
Analytical solution for circular cavity under internal pressure
A book listed the problem infinite circular cavity under internal pressure and said there is a analytical solution for stress and displacements but it didn't give any reference. I have searched many papers and online materials but still couldn't find anything. The closest thing I found...- roger1318
- Thread
- Replies: 1
- Forum: Mechanical Engineering
-
T
Thermo(concept Q, pic included)- How does internal energy increase but
Homework Statement http://img694.imageshack.us/img694/9990/fig24a.jpg" The scenario is, the water in the beaker = system 1. Everything else, including walls = system 2. The bunsen burner heats the water in the beaker. dU or the change in internal energy of system 2 decreases. dT or the...- thechamp
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
T
Analogies for internal resistance and emf
Hi! I'm trying to fully grasp why the potential drops when an internal resistance is present in a source of emf. At first I thought that yes it would be harder to "push" the charges to the higher potential, but once there, why shouldn't the higher potential be the same as before? Just as if a...- The_Lobster
- Thread
- Replies: 4
- Forum: Electrical Engineering
-
J
How come light only reflects off surfaces and not internal planes?
When light approaches and enters a material, why does all the reflection happen at the surfaces? I mean, light is coming at say, some crystalline solid. It hits the first plane of atoms and some is reflected. Some goes into the crystal and passes through the 2nd, 3rd, 4th,..., (n-1)th plane of... -
R
Internal Shear Stress Question
Something has been bugging me for a long time, since I took my first statics class a few years ago. I have a problem with a common figure found in many statics books. I have attached the figure to my post. My question deals with the delta V added to the shear force on the right side of the...- rat4x4
- Thread
-
- Tags
- Internal Shear Shear stress Stress
- Replies: 2
- Forum: Classical Physics