You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Internal: Definition and 991 Discussions
An internality is the long-term benefit or cost to an individual that they do not consider when making the decision to consume a good or service. One way this is related to behavioral economics is by means of the concept of hyperbolic discounting, in which immediate consequences of a decision are disproportionately weighed compared to the future consequences. A potential cause is lack of access to full information regarding the associated costs and benefits prior to consumption. This contrasts with traditional economic theory, which makes the assumption that individuals are rational decision makers who take all personal costs into account when paying for goods and services.One example of a positive internality is the long run effect of exercising, if these are not taken into account when deciding whether to exercise. Future benefits that an individual may not take into consideration include a diminished risk of heart disease and higher bone density. A common example of a potential negative internality is the effect of smoking cigarettes on those who smoke. For the effect of secondhand smoke, see externality. Statistically, 80% of smokers want to quit, and 54% of people who are serious about quitting fail in a week or less. This implies that they do not act in their long-term best interest due to short-term discomfort, also known as a self-control problem. If the demand for cigarettes has a high price elasticity of demand, which evidence seems to suggest, the government can combat the negative internality by raising taxes. It is important to note that elasticity might change based on location and knowledge about the harmful health effects of smoking. In traditional economic theory, a tax diminishes the welfare of the poor because the tax burden shifts to low-income communities, as fewer can afford the good (cigarettes), and horizontal equity (economics) is distorted. However, behavioral economic theory suggests that the tax is not regressive if low-income communities have higher (healthcare) costs and more price sensitivity than individuals with higher incomes. Taxes imposed to combat internalities are most effective when they target a specific good. A tax on junk food could apply to a large variety of goods that are widely consumed, and the cost of the tax might be perceived as more detrimental than beneficial for society. A major issue with creating effective legislature against negative internalities is that the tax imposed should only reflect the cost that individuals do not factor into their consumption decisions. The difficulty in measuring individual knowledge is an obstacle to developing new policies. Another point of concern is that the group benefitting from the tax, such as smokers who want to quit, must be sizable enough to offset any backlash from tobacco companies and lobbyists.
In the following graphs, D' and S' are the demand and supply curves if producers and consumers take all external costs (EC) into consideration. The tax attempting to prevent the internality should be set equal to the difference between D and D' at the optimal quantity, which is the unmeasured internal cost (IC).
View More On Wikipedia.org
In the following graphs, D' and S' are the demand and supply curves if producers and consumers take all external costs (EC) into consideration. The tax attempting to prevent the internality should be set equal to the difference between D and D' at the optimal quantity, which is the unmeasured internal cost (IC).
View More On Wikipedia.org
-
M
Physics -- Change in Internal Energy Help
Homework Statement [/B] Okay guys I have attached a picture of my work. I guess my question really is, if they are telling me that Cp =7/2 am I allowed to assume that I am dealing with a diatomic gas? If so, that would change my equation to (5/2) instead of (3/2) correct? and therefore my...- MauricioValdez
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
T
How does Total Internal Reflection in a mm/sm fiber work?
I know Snell's Law and I do understand that there are single and multimode fibers. A fiber carries light hence to total internal reflection. so far so good. But is it only a single mode fibre that works with total internal reflection? And if so, how does the multimode fibre carry light then? -
R
Change in internal energy when water is heated from 0 to 4c
Homework Statement : [/B]Find the change in internal energy of 2kg water as it is heated from 0°C to 4°C. The specific heat capacity of water is 4200J/Kg and its densities at 0°C and 4°C are 999.9 kg/m3 and 1000kg/m3 respectively. Atm pressure=105PaHomework Equations :ΔU= Q-W W=PΔV M/V=D[/B]The...- randomgamernerd
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-

Internal stresses of an accelerating body
Homework Statement Refer to image attached. Lets say I have a deformable solid that is being accelerated by a force that is equally distributed along the back face of the Main Body that is drawn in the picture. Attached to this Main Body is a Wing. At high accelerations, there will be inertial...- Mike J
- Thread
- Replies: 11
- Forum: Introductory Physics Homework Help
-
L
Finding internal resistance & EMF of a battery in a circuit
Homework Statement When an external resistor of resistance R 1 = 14 Ω is connected to the terminals of a battery, a current of 6.0 A flows through the resistor. When an external resistor of resistance R2 = 64.4 Ω is connected instead, the current is 2.0 A. Calculate the emf and the internal...- lb20
- Thread
- Replies: 11
- Forum: Introductory Physics Homework Help
-
F
Solving an Internal Indeterminate Truss Problem
Homework Statement In this problem , it's a Internal Indeterminate truss . Homework EquationsThe Attempt at a Solution So , I remove one of the redundant member , AC and solve the problem . For the first part , I found the real force first . for the second part , I apply the virtual unit...- fonseh
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
T
Energy Loss During Total Internal Reflection
When a laser beam reflects during total internal reflection, how much of its intensity is lost? I can't the use Fresnel equations as this is for total internal reflection.If you don't know the answer to the above question, what about the same question, but for mirrors instead? What are the... -
C
Internal resistance of a cell varying with power drawn
If we continue to draw a specific amount of power from a cell (re-chargable), how will the internal resistance of the cell vary? Is there any particular mathematical relation between them? Please help me understand- CharanV
- Thread
- Replies: 2
- Forum: Electrical Engineering
-
V
Is internal energy really decreasing in situation 2?
Homework Statement I am trying to understand the law of conservation of energy from a very general perspective but coming across some issues. I am using the equation mentioned at end of this post, which is true for a system with no heat flows into or out of the system. In two situations...- vcsharp2003
- Thread
- Replies: 32
- Forum: Introductory Physics Homework Help
-
M
Internal Combustion Engine question
Just out of curiosity, as I am very amateur here, in ICE could mono-crystal Alumina replace aluminum and create a better engine? Aside from the high cost to manufacture. I know MC-Alumina has a higher density than Al but is considerably stronger. This would be fantastic, I would think for a...- MattMaxwell
- Thread
-
- Tags
- Combustion Engine Internal
- Replies: 3
- Forum: Mechanical Engineering
-
Y
Need some help with forces on a dangling prism Internal Assessment
Homework Statement I made an apparatus of a rectangular prism dangling from a vertical string from one side while the other is touching the ground. The relationship I'am trying to find is between the angle of the prism relative to the ground and the normal force exerted up wards. I am stuck on...- yanshu liang
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
G
Calculating EMF and Internal Resistance: A Practical Guide
Homework Statement I need to calculate EMF and internal resistance from the given resistance and voltage. R = 3 ohms U = 3,6 V EMF- ? I0 - ? r - ? Homework Equations I = U/R EMF = A/q I = EMF/R+r I0 = EMF/r The Attempt at a Solution I = 3,6/3 = 1,2 A I = EMF/R+r 1,2= EMF/3+r ^ I hit a wall...- Gustavs1337
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-

Work done by internal forces on a system of particles
Dear Experts, Trying to analyze the work done by internal forces in a very simple two particle system which is attracted to each other with a constant force, i performed calculations based on two frame of references, 1.The center or mass frame of reference and 2. Frame of reference of one of the...- Nikhil Rajagopalan
- Thread
- Replies: 2
- Forum: Mechanics
-

Calculating Internal Resistance: Methods A and B
Homework Statement [/B] Calculate the internal resistance of V & A based on methods A & B below: Method A Voltmeter = 1.46 V Ammeter = 0.24 A E (emf of battery) = 1.48 V Method B Voltmeter = 1.48 V Ammeter = 0.24 A E (emf of battery) = 1.48 VHomework Equations Ohm's Law: V = I*R The...- Adriano25
- Thread
- Replies: 13
- Forum: Introductory Physics Homework Help
-
R
Thermodynamics Change in Internal Energy?
Homework Statement A closed, rigid tank contains 2 kg of water, initially a two phase liquid–vapor mixture at T1 = 70°C. Heat transfer occurs until the tank contains only saturated vapor at T2 = 120C. Determine the heat transfer for the process, in kJ. answer choices: 3701kJ 119.4kJ 4835kJ...- Ricardeo Xavier
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
D
Thermodynamics -- Internal Energy of Water
Homework Statement Water is initially at P = 1 bar and T = 20°C. 100kg of water is pumped to a higher pressure at which P = 10 bar and T = 25°C. Find ΔU and ΔH Homework Equations H = m*h du = c*dT dh = c*dT + v*dP The Attempt at a Solution So far I have looked in my table and found that at P...- dlacombe13
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
D
Internal Energy Thermodynamics
Homework Statement CO2 is at P=3atm, T = 295K and V=1.2m3. It is isobarically heated to T = 500K. Find ΔU and ΔH Homework Equations dU = cpdT The Attempt at a Solution I am having a hard time in general in this class. I understand that in this problem, ΔP = 0. Does this mean that there must...- dlacombe13
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-

I Prove that V is the internal direct sum of two subspaces
Let V be a vector space. If U 1 and U2 are subspaces of V s.t. U1+U2 = V and U1 and U1∩U2 = {0V}, then we say that V is the internal direct sum of U1 and U2. In this case we write V = U1⊕U2. Show that V is internal direct sum of U1 and U2if and only if every vector in V may be written uniquely...- Austin Chang
- Thread
-
- Tags
- Direct sum Internal Subspaces Sum
- Replies: 7
- Forum: Linear and Abstract Algebra
-

I Internal rotation kinetic energy operator
Hello everybody, My question today is: Given a molecule that has an internal degree of liberty ( let's take the ethane molecule with its internal rotation as an example), how to write the kinetic energy operator by means of the corresponding internal coordinate? Thank you guys. Konte- Konte
- Thread
- Replies: 2
- Forum: Quantum Physics
-

Change in internal energy during water vaporization
According to the first law of thermodynamics, dQ = dU + dW and you can find dU = nCvdT If this is the case then when water at 100°C vaporizes to steam at 100°C shouldn't the change in internal energy be zero because it is dependent on temperature change?- Ian Baughman
- Thread
- Replies: 1
- Forum: Mechanics
-
C
I Accelerating Internal OAM Photon Wavefronts Under Gravity
It is known that wavefronts of internal OAM photons travel slower than light but I wonder what happens if you accelerate such a beam. This should be possible under gravity.- calinvass
- Thread
- Replies: 5
- Forum: Special and General Relativity
-

Joule heating in internal resistances of a solar cell
Hi , How can I calculate the resistive heating occurring in the internal resistances of a solar cell ? Are ohm's law or the power dissipation relation P=RI2 applicable in this case ? if not the case how can we calculate the resistive heating there? Thank you in advance for your help- hikari1987
- Thread
- Replies: 2
- Forum: Electrical Engineering
-
Pin configuration & Internal block diagram of an optocoupler
Homework Statement In that pin configuration, what does the triangle and rectangle in the middle represent?- nothing909
- Thread
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
F
Internal Forces in Truss: Solving for AB at Point A and Point B
Homework Statement For this problem , i found that the internal force AB at point A and point B pointed in the same direction ( as shown) in my working , so , how they cancel off each other ?Since they can't cancel off each other , so they are not stable , right ? The structure is statically...- fonseh
- Thread
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-

Predicting internal temperature with or without HVAC
Hi, how to predict indoor temperature with or without HVAC? I have the internal temperature's value, the external temperature's value and the temperature of HVAC's air output. I want two function, one with HVAC on and other with mode off. Hp: start time 0, end time Tend. Tend has values from 0...- Claude_mar
- Thread
-
- Tags
- Hvac Internal Temperature
- Replies: 2
- Forum: General Engineering
-
S
Work and heat transfer in internal combustion engine
Homework Statement : [/B]At the beginning of the compression stroke of a two-cylinder internal combustion engine the air is at a pressure of 101.325 kPa. Compression redeuces the volume to 1/5 of its original volume, and the law of compression is given by PV^1.2 = const. If the bore and stroke...- Suman_babai
- Thread
- Replies: 5
- Forum: Engineering and Comp Sci Homework Help
-
N
Calculating Internal Energy & Temperature Change of Ideal Gas
Homework Statement What is the change in internal energy (in Joules) of an ideal gas that does 4.675x10^5J of work, while 2.95x10^6J of heat is transferred into the system and 7.95x10^6J of heat is transferred from the system to the environment? Calculate the change in temperature of the two...- Nick88
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
O
Internal Energy of Degenerate Fermi ideal gas to the 4th power
Homework Statement We are asked to derive the expression for the internal energy of an ideal Fermi degenerate gas using Sommerfeld expansions, writing out terms up to the fourth order in ##(\frac{T}{T_F} )## , that is, we must determine ## \alpha ## in the following expression: $$ U=...- Othin
- Thread
- Replies: 7
- Forum: Advanced Physics Homework Help
-
I
Confusion: Internal energy u vs enthelpy h
I've been given the following relations where as I understand it subindex 2 equals subindex e and subindex 1 equals subindex i: ***EDIT*** More accurately subindex 1; initial state of a control mass subindex 2: end state of a control mass (end state is simply state 2 in the problem at hand)...- Imolopa
- Thread
- Replies: 7
- Forum: Thermodynamics
-
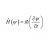
Sublimation: invariant heat or internal energy?
Homework Statement Below, two experiments (1 and 2) are described, in which the same quantity of solid carbon dioxide is completely sublimated, at 25ºC: The process is carried out in a hermetically sealed container, non-deformable with rigid walls; The process is carried out in a cilinder...- Vitor Pimenta
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-

Thin film interference and external / internal reflection
Homework Statement I am working on a thin film interference problem where i have to find the phase difference. What is the relationship between external/internal reflection and the thin film interferenceHomework Equations for external: [/B] for internal: The Attempt at a Solution So far i...- Cocoleia
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Total Internal Reflection / Reflection. What's the answer
Homework Statement Homework EquationsThe Attempt at a Solution https://scontent-kul1-1.xx.fbcdn.net/v/t35.0-12/15146781_1334186669946177_363846788_o.jpg?oh=b75be99f63eba2e70db5e0287581076b&oe=583538C2 The answer given by the book is " Reflection ". But why isn't it Total Internal Reflection ?- SunandaGoh
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
S
A Free theory time-ordered correlation function with two internal fields
Wick's theorem allows one to write a free theory time-ordered ##n##-point correlation function as a product of free theory time-ordered ##2##-point correlation function. The procedure involves the pairwise Wick contraction of fields such that external fields are not paired up each...- spaghetti3451
- Thread
- Replies: 1
- Forum: Quantum Physics
-
B
I Why triatomic gases have internal energy 7RT/2 ?
This table is given in my book, $$\begin{array}[c!c!c!c!] \text{ }&\text{ Transitional }&\text{ Rotational }& \text{ Vibrational} \\ \hline \text{Linear molecules} & 3&2& 3N -5\\ \hline \text{Non-Linear molecules} & 3&3& 3N -6\\ \hline \end{array}$$ It is also given...- Buffu
- Thread
- Replies: 14
- Forum: Atomic and Condensed Matter
-

The internal combustion engine
Homework Statement Hi , I'm struggling with new paragraph about internal engines. When four-stroke internal combustion engine spins crankshaft 100 times , piston does 300 ticks. How many cilinders does engine has? Homework Equations I don't know how to create equation to calculate that , i...- Ugnius
- Thread
-
- Tags
- Combustion Engine Internal
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
I Is it Reasonable to Assume (3/2)*P*V as the Internal Energy of a Real Gas?
I am looking over the kinetic theory of gases. It is most commonly described as U = (3/2)*N*k*T = (3/2)*mass*R*T for a monatomic gas, assuming the gas is ideal. This is based on the derivation, where ultimately (3/2)*P*V = N*K = total kinetic energy of particles. My question, for a real gas...- Matthew Marko
- Thread
- Replies: 5
- Forum: Classical Physics
-

State Functions for Internal Energy and Enthelphy
Hi, As is commonly known, u = u(T,v) h = u(T,p) I've worked with some maths proofs of this a while ago, but do you guys have an intuitive way of understanding this without the maths, that is, why the state function for internal energy is defined by intensive volume and enthalpy with pressure...- Kushwoho44
- Thread
- Replies: 3
- Forum: Mechanical Engineering
-
B
Internal structure of the Earth and it's composition
I was wondering how they managed to know the internal structure of Earth and it's composition while there is lava and it's very hot such that no any instruments can get there...seriously I am confused- bayi
- Thread
- Replies: 8
- Forum: Earth Sciences
-
M
Comparison of internal energies: hot nail versus beaker of water
Homework Statement [/B] A nail is heated in a bunsen burner flame and is about to be dropped into a beaker of water at room temperature. a) Which of the two substances (nail or water) would you expect to initially have: i) the highest internal energy ii) the highest average kinetic...- memeningful
- Thread
-
- Tags
- Comparison Energies Hot Internal Water
- Replies: 4
- Forum: Introductory Physics Homework Help
-

I Can internal energy be calculated from equation of state?
We know, $$dU=TdS-PdV$$ ##\int PdV## can be calculated if the equation of state is given. I tried to express ##S## as a function of ##P ,V## or ##T## (any two of those). $$dS=\left(\frac{\partial S}{\partial V}\right)_T dV+\left(\frac{\partial S}{\partial T}\right)_V dT$$ $$=\left(\frac{\partial...- arpon
- Thread
- Replies: 2
- Forum: Other Physics Topics
-

Internal resistance of each cell+energy dissipated in 1 min
Homework Statement A bulb is used in a torch which is powered by two identical cells in series each of EMF 1.5 V. The bulb then dissipates power at the rate of 625 mW and the PD across the bulb is 2.5 V. Calculate (i) the internal reistance of each cell and (ii) the energy dissipated in each...- moenste
- Thread
- Replies: 20
- Forum: Introductory Physics Homework Help
-

Internal resistance of the battery and energy transformed
Homework Statement The battery in the circuit below has EMF 5.4 V and drives a current of 0.30 A through a lamp. The voltmeter reading is 4.8 V. Explain why the voltmeter reading is less than the EMF of the cell. Calculate values for (a) the internal resistance of the battery, and (b) the...- moenste
- Thread
- Replies: 14
- Forum: Introductory Physics Homework Help
-
E
How much of the atom's internal energy is released?
Homework Statement A uranium-238 atom can break up into a thorium-234 atom and a particle called an alpha particle, α-4. The numbers indicate the inertias of the atoms and the alpha particle in atomic mass units (1 amu = 1.66 × 10−27 kg). When an uranium atom initially at rest breaks up, the...- emily081715
- Thread
- Replies: 22
- Forum: Introductory Physics Homework Help
-

When can internal reflection be used to find the index of refraction
Homework Statement I need to describe an experiment in which I would use internal reflection to determine the index of refraction of material Homework Equations Snell's law The Attempt at a Solution I understand the concept of internal reflection, such as the critical angle etc. I am just...- Cocoleia
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
J
Calculating Total Internal Energy for a Monatomic Gas at Constant Pressure
Homework Statement There is a monatomic gas held at a constant pressure of P = 1.48-atm, it also has a molar mass M = 16-g/mol and density ρ =1.9 × 10-3-g·cm-3. Find the total internal energy of 1-mol of this gas. Homework Equations U = Q + W E = nCvT PV = nRT The Attempt at a Solution I...- john mcgrain
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
S
Change in the internal energy of an isobaric process
Homework Statement A cylinder contains 0.250mol of carbon dioxide (CO2) gas at a temperature of 27.0∘C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00atm on the gas. The gas is heated until its temperature increases to 127.0∘C. Assume that the...- Stendhal
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Does Decreasing Volume and Internal Energy of a Gas Affect Heat Flow and Constant Pressure?
A gas in a cylinder with constant pressure, the gas cooled down and its internal energy decreased as well as its volume. The heat Q will be flowing into the gas or out of the gas? My try for the solution: As the volume decrease the work done by the gas will be negative. The gas cooled down, so...- Mohammed Alqadhi
- Thread
- Replies: 7
- Forum: Thermodynamics
-
I
Where in the brain do we sense our internal organs?
Hello, I know that the sensory cortex in the postcentral gyrus collects sensory information from different parts of the body, but in all the drawings and explanations, I see that it only gathers information from the surface of the body. Which part of the brain gathers information from our...- icakeov
- Thread
- Replies: 8
- Forum: Biology and Medical
-
N
I Help understanding internal energy
Edit: @Dale managed to do a far better job in stating the problem, essentially the question is why do we get the same internal energy for different microstates corresponding to a single thermodynamic state Original Post: So I'm self studying a course about thermodynamics and statistical...- nashed
- Thread
- Replies: 6
- Forum: Other Physics Topics
-
J
Finding Internal Resistance of a Circuit
Homework Statement See image attached. I am currently stuck on part b. Homework Equations (Rt) (Rint) / Rt + (Rint) The Attempt at a Solution I thought that Rt would be 26.7 kohms, but my answer is not matching up with the answer in the book. I am unsure why since the resistors are in...- Josh225
- Thread
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help