- #1
AdityaDev
- 527
- 33
I found this in "March's Advanced organic chemistry"
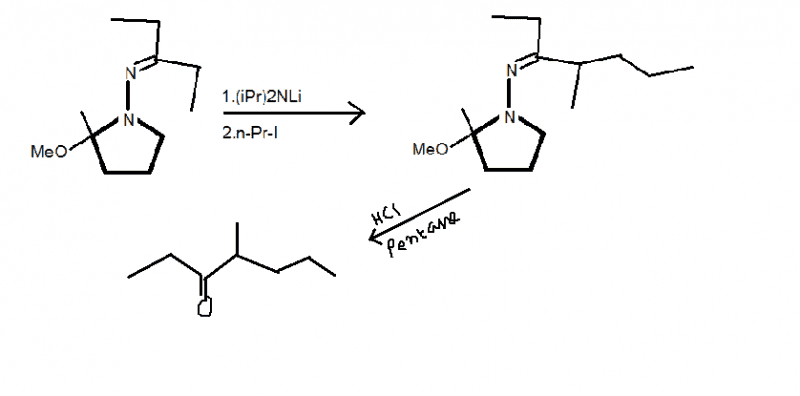
After that step, on using HCl in pentane, you will get 4-methyl-3-Heptanone.
So this method was used to convert achiral 2-pentanone to a chiral compound, by first converting it to the compound on left by addition reaction and then using a 2 step process. On checking the appendix, its given that the reaction is alkylation of imines.
Can someone give me the mechanism for alkylation of imines using iPr2NLi?
And what's the use of pentane with HCl?
After that step, on using HCl in pentane, you will get 4-methyl-3-Heptanone.
So this method was used to convert achiral 2-pentanone to a chiral compound, by first converting it to the compound on left by addition reaction and then using a 2 step process. On checking the appendix, its given that the reaction is alkylation of imines.
Can someone give me the mechanism for alkylation of imines using iPr2NLi?
And what's the use of pentane with HCl?