- #1
rwooduk
- 762
- 59
I am seeing from my results that an oxidation process is taking place, the pollutants degradation correlates closely with the formation of radicals.
However, in one case there are less radicals produced and there is no degradation...
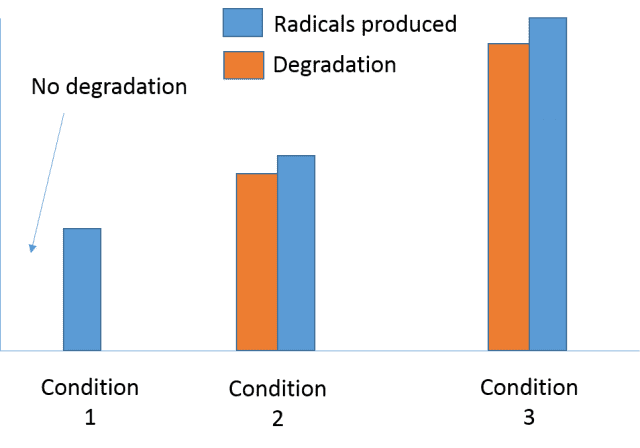
I would like to ask if on occasions there must be a certain number of radicals to attack the pollutant? Or it is likely due to the change in conditions?
Thanks again for any help / ideas on this (!)
However, in one case there are less radicals produced and there is no degradation...
I would like to ask if on occasions there must be a certain number of radicals to attack the pollutant? Or it is likely due to the change in conditions?
Thanks again for any help / ideas on this (!)