- #1
Guest432
- 48
- 2
Hello all!
In my Nuclear Power assignment I decided to analyse this graph:
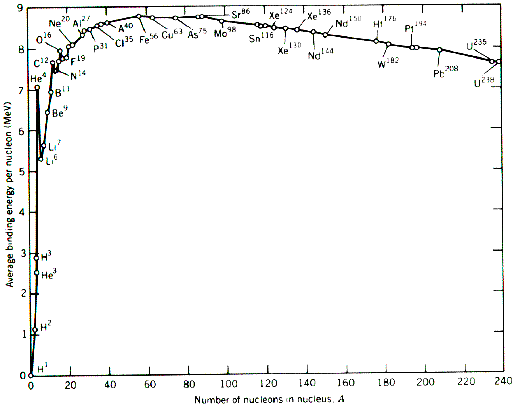
I mention that
"The difference in atomic mass and binding energy per nucleon for deuterium and helium (fusion elements) is ≈3u and 5.96 MeV respectively. However, for all elements past Iron (fission elements) the difference in any two binding energies is < 1.3 MeV. Such a vast energy yield, even in comparison to fission, is the reason why obtaining net power from fusion is such a holy grail."
However, I am perplexed as to why exactly the difference in binding energy of elements before Iron is so high! I have discussed the fact neutrons increase the distance between protons in nuclei, so I am taking a guess that elements with a low atomic mass have less neutrons, ergo more repulsive force and greater binding energy. However, this logic seems weak and doesn't really explain why the trend of binding energy per nucleon is what it is.
Thanks :)
In my Nuclear Power assignment I decided to analyse this graph:
I mention that
"The difference in atomic mass and binding energy per nucleon for deuterium and helium (fusion elements) is ≈3u and 5.96 MeV respectively. However, for all elements past Iron (fission elements) the difference in any two binding energies is < 1.3 MeV. Such a vast energy yield, even in comparison to fission, is the reason why obtaining net power from fusion is such a holy grail."
However, I am perplexed as to why exactly the difference in binding energy of elements before Iron is so high! I have discussed the fact neutrons increase the distance between protons in nuclei, so I am taking a guess that elements with a low atomic mass have less neutrons, ergo more repulsive force and greater binding energy. However, this logic seems weak and doesn't really explain why the trend of binding energy per nucleon is what it is.
Thanks :)
Last edited: