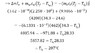- #1
ayans2495
- 58
- 2
- Homework Statement
- A heated block of iron at temperature Ti with a mass of 61.331g is submerged into a beaker of water which has a mass of 200.562g and an inital temperature of 24.6 degrees Celsius. Once the iron is immersed into the water, 0.362 grams of the water evaporates. What is the initial temperature Ti of the iron block such that the final equilibrium temperature Tf is 34.3 degrees celsius?
- Relevant Equations
- Q=ml
Q=mcΔT Note: Δm=0.632