- #1
Ayman Saif
- 2
- 0
Hello,
according to the lead acid battery standard equations
The overall chemical reaction is:
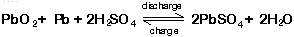
At the negative terminal the charge and discharge reactions are:
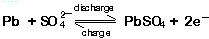
At the positive terminal the charge and discharge reactions are:
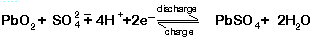
My Q?
If we empty all liquid from old battery and add H2O (water)
then charge with reverse polarity
can we get pure PB?
according to the lead acid battery standard equations
The overall chemical reaction is:
At the negative terminal the charge and discharge reactions are:
At the positive terminal the charge and discharge reactions are:
My Q?
If we empty all liquid from old battery and add H2O (water)
then charge with reverse polarity
can we get pure PB?