- #1
rwooduk
- 762
- 59
Does anyone here have any analytical experience with dissolved gas, particularly CO2?
When CO2 is dissolved in water there can be several reactions:
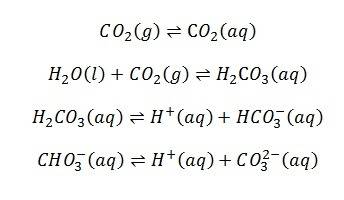
What I would ideally like to do is dissolve CO2 into water and then by some means of analysis, see how much of each species there is in the water. However, carbonic acid seems very difficult to detect, see:
http://www.chemistryviews.org/details/ezine/973843/Tracking_Carbonic_Acid.html
I could use a bicarbonate indicator for the bicarbonate but this is just a colour change and won't give an amount. Hydrogen carbonate seems to be the same as bicarbonate so I've no idea how I would differentiate between the two. And also I have no idea on the carbonate.
Would these things even exist in stable amounts of would they keep reacting and reversibly reacting?
If someone could point me in the right direction, or just give me a place to start it would really be appreciated as this really isn't my field (not a chemist).
I have access to HPLC, GC etc etc
When CO2 is dissolved in water there can be several reactions:
What I would ideally like to do is dissolve CO2 into water and then by some means of analysis, see how much of each species there is in the water. However, carbonic acid seems very difficult to detect, see:
http://www.chemistryviews.org/details/ezine/973843/Tracking_Carbonic_Acid.html
I could use a bicarbonate indicator for the bicarbonate but this is just a colour change and won't give an amount. Hydrogen carbonate seems to be the same as bicarbonate so I've no idea how I would differentiate between the two. And also I have no idea on the carbonate.
Would these things even exist in stable amounts of would they keep reacting and reversibly reacting?
If someone could point me in the right direction, or just give me a place to start it would really be appreciated as this really isn't my field (not a chemist).
I have access to HPLC, GC etc etc