- #1
songoku
- 2,294
- 325
- Homework Statement
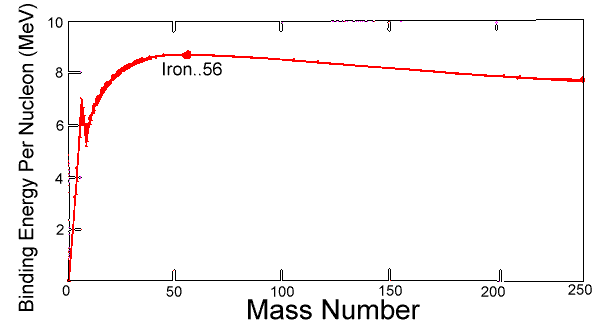
- The graph below shows how the binding energy per nucleon of a nucleus varies with mass number A
(graph is given below)
Let region P is around mass number equals to 5, region Q is around mass number equals to 50 and region R is around mass number equals to 200
Which one of the following statements is not true?
a. The binding energy per nucleon increases most significantly at lower nucleon numbers
b. Nuclei in region Q are more stable than nuclei in region R
c. Nuclear fusion reaction bring nuclei closer to region Q
d. Energy is released in nuclear fission reactions from nuclei in region R
- Relevant Equations
- Not sure
Option A is correct because the graph increases sharply at lower nucleon number
Option B is correct because higher binding energy per nucleon means more stable
Option C is correct because either fusion or fission will bring the product to Q
So my guess the answer is D but I do not understand why
Thanks
Option B is correct because higher binding energy per nucleon means more stable
Option C is correct because either fusion or fission will bring the product to Q
So my guess the answer is D but I do not understand why
Thanks