- #1
Sunwoo Bae
- 60
- 4
- Homework Statement
- For the ion shown below, draw the most important resonance forms and predict which resonance form is likely to be the major contributor.
- Relevant Equations
- none
ion shown:
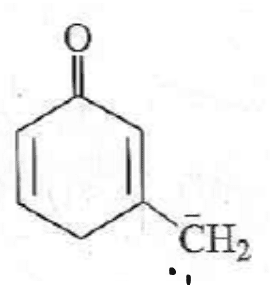
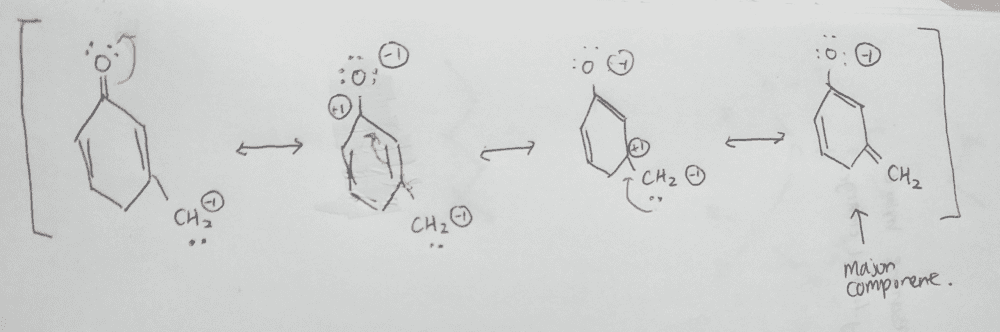
My answer:
the following is the answer of the question:
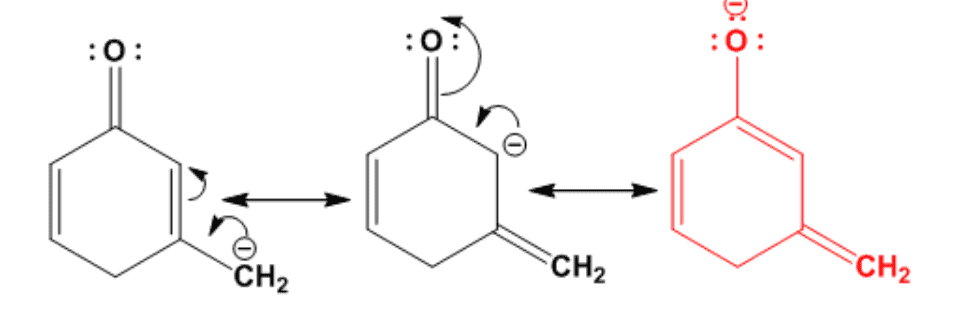
I identified the major contributor correctly, but the resonance structures, but the second resonance I drew is missing in the answer sheet.
Can anyone tell me why the second resonance structure is not part of the answer?
Thank you
My answer:
the following is the answer of the question:
I identified the major contributor correctly, but the resonance structures, but the second resonance I drew is missing in the answer sheet.
Can anyone tell me why the second resonance structure is not part of the answer?
Thank you