- #1
tanaygupta2000
- 208
- 14
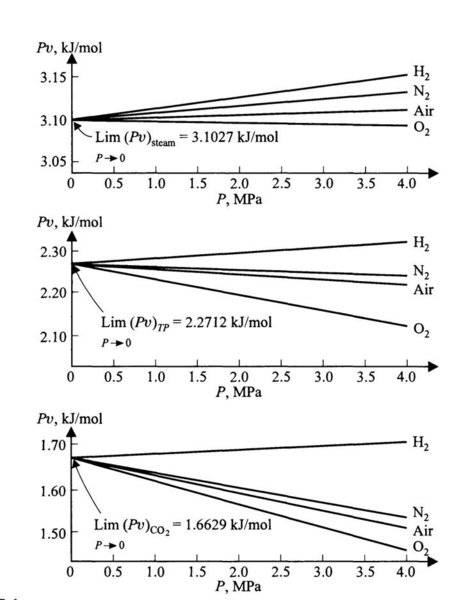
In the experimental virial equation for real gases,
Pv = A(1+BP) (for small values of P)
as 'P' approaches zero, then why do we get some finite value of 'Pv' instead of approaching to zero value, since 'Pv' is directly proportional to 'P' ?
{P = Pressure of gas, v = Molar volume of gas, A,B = Virial Constants}
Pv = A(1+BP) (for small values of P)
as 'P' approaches zero, then why do we get some finite value of 'Pv' instead of approaching to zero value, since 'Pv' is directly proportional to 'P' ?
{P = Pressure of gas, v = Molar volume of gas, A,B = Virial Constants}
