- #1
feynman1
- 435
- 29
The left pic is the initial state and the right pics are 2 different descriptions for a metal under electric field E. Are the 2 on the right contradictory and which is correct?
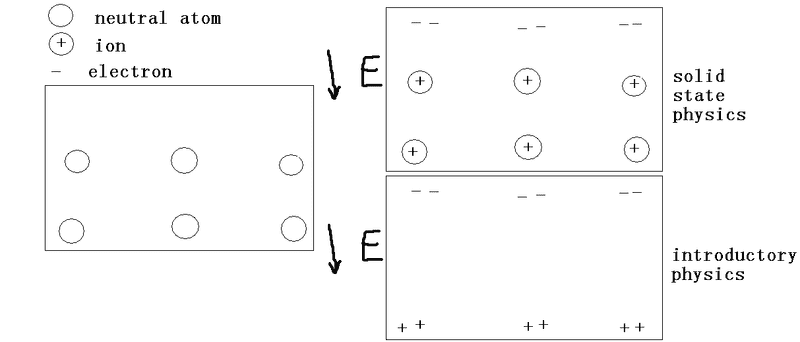
The bottom pic can be seen in any introductory physics textbook. The top is drawn by myself, no reference.berkeman said:Can you post links to the references where you read about those models? Thanks.
The bottom pic means that electrons are pushed by E to the top, leaving the bottom boundary carrying positive charge. Equations can be coulomb forces.berkeman said:That's not very helpful I'm afraid. What motivated you to draw the bottom picture? What equations are you assuming while making the drawings?
Ok, so you drew the top left picture labeled "solid state physics" yourself. Can you explain how, according to that picture, the electric field inside the conductor is zero? In the "introductory physics" picture, it's easy. The positive and negative charges are on the surface of the conductor. The negative charges "stop" the electric field lines at one surface and "start" them at the other. This does not seem to be the case in the "solid state" picture. If one draws a Gaussian surface around the positive charges, there will be net electric flux out of it which means there is an electric field inside the conductor under static conditions. That is turn means that the conductor is not an equipotential.feynman1 said:The bottom pic can be seen in any introductory physics textbook. The top is drawn by myself, no reference.
I thought of what you say and totally agree all along. But the ions in the middle of a solid should be fixed in place so where can they go?kuruman said:Ok, so you drew the top left picture labeled "solid state physics" yourself. Can you explain how, according to that picture, the electric field inside the conductor is zero? In the "introductory physics" picture, it's easy. The positive and negative charges are on the surface of the conductor. The negative charges "stop" the electric field lines at one surface and "start" them at the other. This does not seem to be the case in the "solid state" picture. If one draws a Gaussian surface around the positive charges, there will be net electric flux out of it which means there is an electric field inside the conductor under static conditions. That is turn means that the conductor is not an equipotential.
I don't get what you mean. Do you think the right pics are correct or not?caz said:In a real conductor, there are so many electrons that even for a strong E field, there is not a lot of displacement so the continuum model for a conductor is valid.
Yes, the positive ions are indeed fixed but the electrons are not. Electrons will be attracted to the fixed positive ions and neutralize them. In the "solid state" drawing the row in the middle should have neutral atoms, not charged ions.feynman1 said:I thought of what you say and totally agree all along. But the ions in the middle of a solid should be fixed in place so where can they go?
Gauss' law will require this to happen. But from the opinion of force analysis, why would electrons be attracted to the ions in the middle and end up staying there rather than driven by E all the way to the top boundary?kuruman said:In the "solid state" drawing the row in the middle should have neutral atoms, not charged ions.
Electrons move in response to external electric fields. The electric field inside the conductor is the vector sum of the external electric field ##\vec E_{ext.}## and the induced field ##\vec E_{ind.}## that is produced by the movement of electrons to the surface. These two fields cancel each other inside the conductor. The cancellation occurs because the free electrons will move and keep on moving until they have no more reason to move which happens when the field everywhere inside the conductor goes to zero. The solid state physics drawing that you drew has positive ions inside the conductor which means that there is a non-zero field in their vicinity. Therefore electrons will move to neutralize these as well as any other positive ions inside the surface of the conductor. This leaves positive ions only on the surface.feynman1 said:Gauss' law will require this to happen. But from the opinion of force analysis, why would electrons be attracted to the ions in the middle and end up staying there rather than driven by E all the way to the top boundary?
The ions in the middle want to absorb the electrons, but what if E successfully drives the electrons upwards with a larger force without letting them reach and neutralize the ions in the middle?kuruman said:The solid state physics drawing that you drew has positive ions inside the conductor which means that there is a non-zero field in their vicinity. Therefore electrons will move to neutralize these as well as any other positive ions inside the surface of the conductor. This leaves positive ions only on the surface.
Then how should a better pic look like?caz said:I think the first picture is misleading. It over emphasizes the number of electrons at the surface compared to the actual number of electrons.
I drew only a few ions for illustration, not accurate.caz said:There should be a bunch of nuclei with electrons in the middle so that the “surface like” charge is apparent.
I didn't mix classical with quantum. I use classical only.Vanadium 50 said:(1) About that user name. I met Feynman. You're no Feynman.
(2) The fact that you couldn't give an answer to @berkeman 's entirely reasonable question of where you read this is telling. It suggests the misunderstanding lies with you can not the textbooks. I know of no textbook that says positive ions in a metal move in an electric field (your bottom drawing). If you know of one, say so, not just the vague and unactionable (and likely incorrect) "any introductory physics textbook."
(3) You keep groping towards the century-discredited Drude model, where electrons behave as a classical gas. As we said in your last thread on this, a classical theory of electromagnetism, where charge is treated as a continuous fluid will work (in its domain of validity). A quantum-mechanical treatment of electrons will work. Mixing the two, as you are trying to do, will not work. A classical treatment of electrons, inherently a quantum mechanical object, cannot give the right answer.
You're talking about charged particles moving within a solid. That is quantum, not classical.feynman1 said:I didn't mix classical with quantum. I use classical only.
Why is there no ions or electrons? Electrons flow in such a fluid and collide with ions?Nugatory said:There is a classical model, valid with its domain of applicability, in which the charge behaves like a continuous fluid... but in that model there are no electrons and ions.
Of course electrons and ions are physically present, but the relevant classical theory treats the charge distribution as continuous so there are no electrons and ions in the classical description. The charge distribution is a fluid in this theory, insted of a collection of charged particles.feynman1 said:Why is there no ions or electrons? Electrons flow in such a fluid and collide with ions?
Did this fluid theory mention what happens when such a fluid (electrons) hits the metal boundary? Will the fluid immediately lose its velocity?Nugatory said:Of course electrons and ions are physically present, but the relevant classical theory treats the charge distribution as continuous so there are no electrons and ions in the classical description. The charge distribution is a fluid in this theory, insted of a collection of charged particles.
Introductory physics focuses on the fundamental principles and laws of motion, energy, and matter, while solid state physics deals with the behavior of matter in its solid form, specifically at the atomic and molecular level.
The gap exists because solid state physics requires a deeper understanding of quantum mechanics and statistical mechanics, which are not typically covered in introductory physics courses. Additionally, solid state physics involves more complex mathematical concepts and experimental techniques.
Solid state physics has numerous practical applications, including the development of electronic devices such as transistors, computer chips, and solar cells. It also plays a crucial role in materials science and engineering, as well as in the study of condensed matter and nanotechnology.
Solid state physics can be challenging due to its reliance on advanced mathematical concepts and its interdisciplinary nature. However, with dedication and a strong foundation in introductory physics and mathematics, it is certainly possible to grasp the fundamental principles and applications of solid state physics.
To bridge the gap, it is important to have a strong understanding of introductory physics principles and mathematical concepts such as calculus, linear algebra, and differential equations. It is also helpful to take additional courses in quantum mechanics and statistical mechanics, and to engage in hands-on laboratory experiences to gain a better understanding of experimental techniques used in solid state physics research.