- #1
nightingale
- 53
- 1
Homework Statement
The exhaust gases from a natural gas-fired central heating boiler leave the boiler at a temperature of 70 deg C. The oxygen content of the exhaust gases is 9% by volume (dry). The rate of energy input (Gross calorific value) to the boiler from natural gas is 16kW. The gross calorific value of natural gas is 55MJ/kg. For the purposes of all calculations assume that natural gas is 100% Methane (CH4). The pressure in the combustion chamber of the boiler and throughout the heat recovery system is 1 bar.
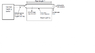
The exhaust gases are blown down a plastic pipe situated in a room are then exhausted to atmosphere, as shown in the diagram attached. The warm pipe provides a heat input to the room of 0.75kW. The pipe is 100mm in diameter and is made up of a number sections 1m in length.
The overall heat transfer coefficient (U) for heat transfer from the exhaust gases through the pipe to the room is 9.5 W/m^2K. The room temperature is 20 deg C.
Calculate:
1. The temperature to which the exhaust gases must be cooled in order to recover 0.75kW of heat.
2. The number of 1m length of pipe needed to provide the heat input of 0.75kW to the room.
Homework Equations
Hints:
The best way to calculate the final temperature of the exhaust gases is to consider cooling down to a different temperatures and identifying (by iterating between the nearest values) which temperature gives 0.75kW of heat recovery.
To calculate the heat recovery from the exhaust gases as they cool through a temperature difference of Delta T, use Q = mCpDeltaT for the dry products of combustion and Q = mDeltah for the water vapour, where appropriate enthalpy values are taken from tables. (A temperature interval of about 5 deg C is appropriate).
As the exhaust gas temperature varies along the pipe, the best way to calculate the length needed is to consider each temperature interval, assume a mean gas temperature, then calculate the length of the pipe needed to give the heat transfer between the exhaust gas and the room as the gas cools over the temperature interval.
The Attempt at a Solution
CH4 + x 02 + 3.76x N2 = a CO2 + b H20 + c 02 + d N2
a = 1
b = 2
x = 2 + c
d = 3.76x = 3.76(2 + c) = 7.52 + 3.76c
9% oxygen, dry
0.09 = c / (a+c+d)
0.09 = c/(1+c+7.52 + 3.76c)
c = 1.3417 moles
and thus x = 3.417 moles
Reactant
mass (Molar Mass * moles)
CH4 1 moles 16 * 1 = 16kg
Products
mass (Molar Mass * moles) mass/kg fuel
CO2 1 mole 44*1 = 44kg 44/16 = 2.75kg/kg fuel
H2O 2 moles 18*2 = 36 kg 36/16 = 2.25kg/kg fuel
O2 1.3417 moles 32*2 = 42.9344kg 42.934/16 = 2.6834kg/kg fuel
N2 12.565 moles 28*12.565 = 351.82 kg 351.82/16 = 22kg/kg fuel
I am completely clueless on how to continue afterwards. The hint told me to make an assumptions of the final temperature that will allow a heat recovery of 0.75 kW.
By using the formula:
Q = ∑(mass of dry product*Cp* ΔT) + mass of H2O * enthalpy of steam at 70 deg C - (mass of H20 water * enthalpy of water at assumed Temp + mass of H2O vapour * enthalpy of steam at assumed temperature)
I found the mass of water= 0.0985kg and the water vapour= 0.110864kg.
I work on excel spread sheet, taking the assumptions of the temperature from 0 deg to 35 deg, all give me the answer of more than 0.75 kW, the required output. At 1.5 deg, the heat recovery is found to be 7510kW, an outrageous amount compared to the required 0.75kW. Could you please help?
Thank you in advance.
 I see what you do and the column I should have looked at is P, and it's fine up to the onset of condensation. I won't interrupt any more and watch you and Chet at work :)
I see what you do and the column I should have looked at is P, and it's fine up to the onset of condensation. I won't interrupt any more and watch you and Chet at work :)