- #1
maistral
- 240
- 17
- TL;DR Summary
- Derivation of formula.
Hi, I have been reading a few literature regarding excess Helmholtz energy and I encountered this definition from the paper of Wong and Sandler (apparently, from the mixing rule used in a EOS):
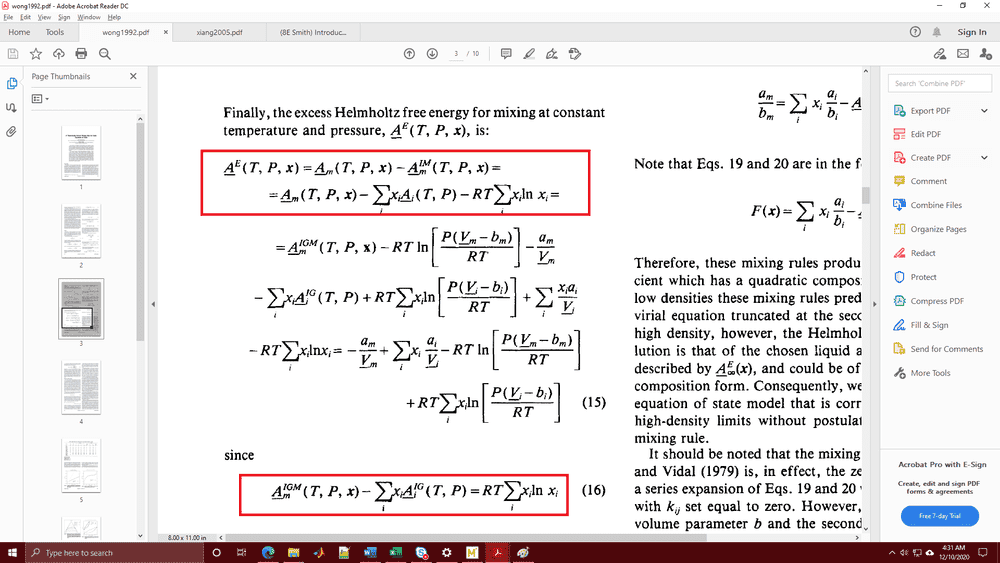
In particular, the ones in the red boxes.
How did these equations come into being? I tried to look for it in books like Tester and SVNA and I still am unable to find where on Earth did these come from. Can someone point me to a reliable resource? Thanks.
In particular, the ones in the red boxes.
How did these equations come into being? I tried to look for it in books like Tester and SVNA and I still am unable to find where on Earth did these come from. Can someone point me to a reliable resource? Thanks.