- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Help with finding chirality centres
- Thread starter andrewvidler
- Start date
-
- Tags
- Chirality
In summary, the conversation is about identifying chiral centres in complex molecules. The basic rule for recognizing chiral carbons is a carbon with four different substituents. However, in complex molecules with multiple chiral centres, this rule may not be easy to apply. To identify chiral centres, one can start by selecting an atom and comparing the atoms on each side of it. If they lead to the same place and there are no differences on the way, then the atom is not chiral. It is recommended to practice on simple molecules before moving on to more complex ones. Additionally, it is important to note that the distance along a separate branch from the centre to a different substituent should be considered when determining chiral centres.
Physics news on Phys.org
- #2
Borek
Mentor
- 28,951
- 4,245
What methods of recognizing chiral carbons do you know?
Please note that attaching .doc files is not a good idea - many people are reluctant to open them, as they can contain viruses. In such a case best approach is to attach an image.
Edit: I think I am bored, I have replaced doc with png in your post.
Please note that attaching .doc files is not a good idea - many people are reluctant to open them, as they can contain viruses. In such a case best approach is to attach an image.
Edit: I think I am bored, I have replaced doc with png in your post.
- #3
andrewvidler
- 5
- 0
- #4
Borek
Mentor
- 28,951
- 4,245
Perhaps you are thrown off by cycles?
Select an atom, move out from it in each direction and compare atoms on the way. If you get to the same place from both sides and there were no differences on the way - atom is not chiral.
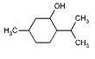
Try it on some simple molecules first, say cyclohexanol and 2-methylcyclohexanol.
Select an atom, move out from it in each direction and compare atoms on the way. If you get to the same place from both sides and there were no differences on the way - atom is not chiral.
Try it on some simple molecules first, say cyclohexanol and 2-methylcyclohexanol.
- #5
andrewvidler
- 5
- 0
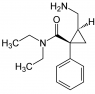
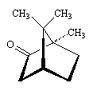
Hi - how about this one? I am confused as to what constitutes a different group. I mean how far do go along the separate branches from the ceontre to say that is a differernt substituent.
this is one where it days there are 3. I know where they are but i don't see why
this is one where it days there are 3. I know where they are but i don't see why
Attachments
- #6
Borek
Mentor
- 28,951
- 4,245
andrewvidler said:how far do go along the separate branches from the centre to say that is a differernt substituent
Either till you "meet" or till you find a difference - whichever comes first.
1. What is a chirality centre?
A chirality centre, also known as a stereocenter, is an atom in a molecule that has four different groups attached to it, resulting in the molecule having two non-superimposable mirror images. These mirror images are known as enantiomers.
2. How do I identify chirality centres in a molecule?
To identify chirality centres, you must first determine the molecule's molecular formula and draw its structural formula. Then, look for carbon atoms that have four different groups attached to them. These carbon atoms are the chirality centres.
3. Why is it important to know the chirality centres in a molecule?
The chirality centres in a molecule determine its optical activity, which is the ability to rotate the plane of polarized light. This is important in pharmaceuticals, as only one enantiomer of a drug may be effective, while the other could have harmful side effects. Knowing the chirality centres can also help predict the molecule's reactivity and biological activity.
4. How can I determine the absolute configuration of a chirality centre?
The absolute configuration of a chirality centre can be determined by using the Cahn-Ingold-Prelog (CIP) rules. These rules assign priority to the four groups attached to the chirality centre based on the atomic number of the atoms bonded to the chirality centre. The group with the highest atomic number is given the highest priority, and the lowest priority group is placed in the back. The remaining two groups are then compared, and the molecule is rotated until the second and third highest priority groups are in a descending order.
5. How does the presence of chirality centres affect a molecule's physical properties?
The presence of chirality centres can affect a molecule's physical properties, such as its melting point, boiling point, and solubility. Enantiomers have different physical properties, such as different melting points and solubilities, due to their different spatial arrangements. This can be useful in separating and purifying enantiomers in industries such as pharmaceuticals and agrochemicals.
Similar threads
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 2K
Chemistry
A dilemma with stereochemistry
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 3K
-
General Math
- Replies
- 1
- Views
- 997
-
Biology and Chemistry Homework Help
- Replies
- 15
- Views
- 3K
-
Atomic and Condensed Matter
- Replies
- 8
- Views
- 1K
-
High Energy, Nuclear, Particle Physics
- Replies
- 13
- Views
- 2K
-
Quantum Physics
- Replies
- 6
- Views
- 781
-
Biology and Chemistry Homework Help
- Replies
- 2
- Views
- 2K
-
High Energy, Nuclear, Particle Physics
- Replies
- 4
- Views
- 1K
Share: