- #1
ChiralSuperfields
- 1,216
- 132
- Homework Statement
- Please see below
- Relevant Equations
- Please see below
For this,
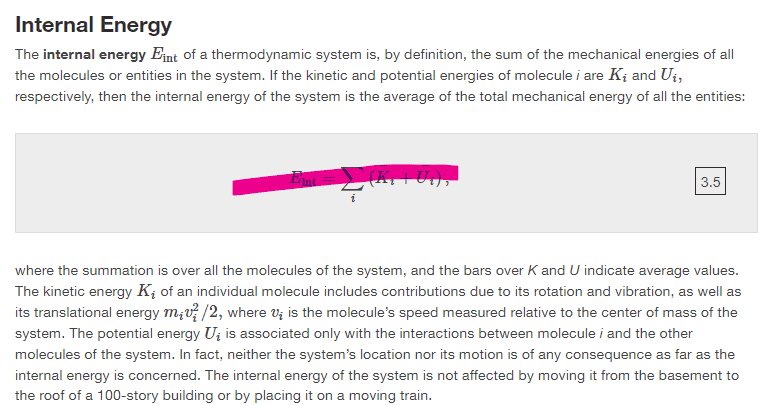
They say internal energy is the sum of the all the mechanical energies of each particle in within the thermodynamic system, however, they then define internal energy differently using the average mechanical energy for all particles within the system (Pink equation). Does someone please know why they did that?
Many thanks!
They say internal energy is the sum of the all the mechanical energies of each particle in within the thermodynamic system, however, they then define internal energy differently using the average mechanical energy for all particles within the system (Pink equation). Does someone please know why they did that?
Many thanks!