- #1
agoogler
- 81
- 0
What are the rules for choosing main branch ?
Wikipedia states -
"Identification of the parent hydrocarbon chain. This chain must obey the following rules, in order of precedence:
1 It should have the maximum number of substituents of the suffix functional group. By suffix, it is meant that the parent functional group should have a suffix, unlike halogen substituents. If more than one functional group is present, the one with highest precedence should be used.
2 It should have the maximum number of multiple bonds
3 It should have the maximum number of single bonds.
4 It should have the maximum length."
I don't understand how 3 and 4 differ. Which single bonds are we counting ? only carbon-carbon single bonds in the main chain right?
Also , i heard there is a rule that says if you've two equal length chains , then choose the more substituted one. Why is it not given here?
And now now imagine you've two choices for a chain , like this -
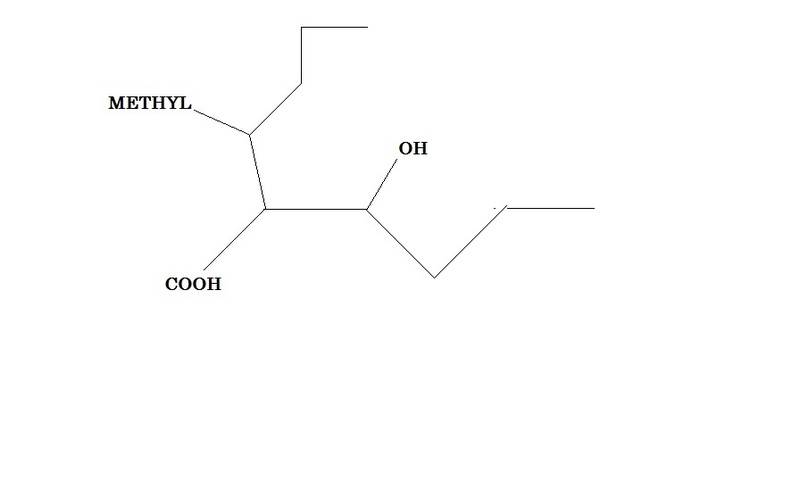
The chemdoodle program (http://web.chemdoodle.com/demos/iupac-naming) gives the name as 2-(1-Hydroxybutyl)-3-methylhexanoic acid . I simply do not understand why the methyl substituted chain is chosen instead of hydroxy substituted one and how that fits with iupac rules given in wikipedia.
Please help.
Wikipedia states -
"Identification of the parent hydrocarbon chain. This chain must obey the following rules, in order of precedence:
1 It should have the maximum number of substituents of the suffix functional group. By suffix, it is meant that the parent functional group should have a suffix, unlike halogen substituents. If more than one functional group is present, the one with highest precedence should be used.
2 It should have the maximum number of multiple bonds
3 It should have the maximum number of single bonds.
4 It should have the maximum length."
I don't understand how 3 and 4 differ. Which single bonds are we counting ? only carbon-carbon single bonds in the main chain right?
Also , i heard there is a rule that says if you've two equal length chains , then choose the more substituted one. Why is it not given here?
And now now imagine you've two choices for a chain , like this -
The chemdoodle program (http://web.chemdoodle.com/demos/iupac-naming) gives the name as 2-(1-Hydroxybutyl)-3-methylhexanoic acid . I simply do not understand why the methyl substituted chain is chosen instead of hydroxy substituted one and how that fits with iupac rules given in wikipedia.
Please help.