- #1
Piotrovskiy Yury
- 6
- 0
- TL;DR Summary
- Kelvin - not the basic, but the derived unit of measurement.
1. Kelvin - not the basic, but the derived unit of measurement?
Modern physics believes that kelvin is the basic unit of measurement of SI (one of seven).
At the same time, in all encyclopedias and textbooks it is written:
T= Θ/k = Θ /1,380649x10е-23
Θ - is the energy of the molecule in joules.
k - is the Boltzmann constant = 1,380649e-23 J / K
T - is the absolute temperature in kelvin.
From the formula it follows that the absolute temperature of kelvin is derived through the joules of kinetic energy of the molecule.
And this means that Kelvin is not the basic unit, but a derived unit.
Another proof that kelvin is not the basic, but a derivative, is a site-converter
Convert kelvin [K] <-> joule [J]
https://www.translatorscafe.com/unit-converter/EN/...
2. In accordance with the fact that kelvin is not the basic unit, it is proposed to legalize, along with the use of the Celsius scale and others, the mandatory parallel use of the energy scale in joules.
That is, in addition to “historically established practice”, habits, inertia of thinking, temperature measurement in degrees, not joules, is unjustified.
In addition, the measurement of temperature in degrees (Celsius, Kelvin, Fahrenheit, etc.) hides, disguises the essence of temperature as a physical phenomenon, complicates its understanding and contributes to the physical illiteracy of the people.
THERMOMETERS WITH JOULE - SCALE:
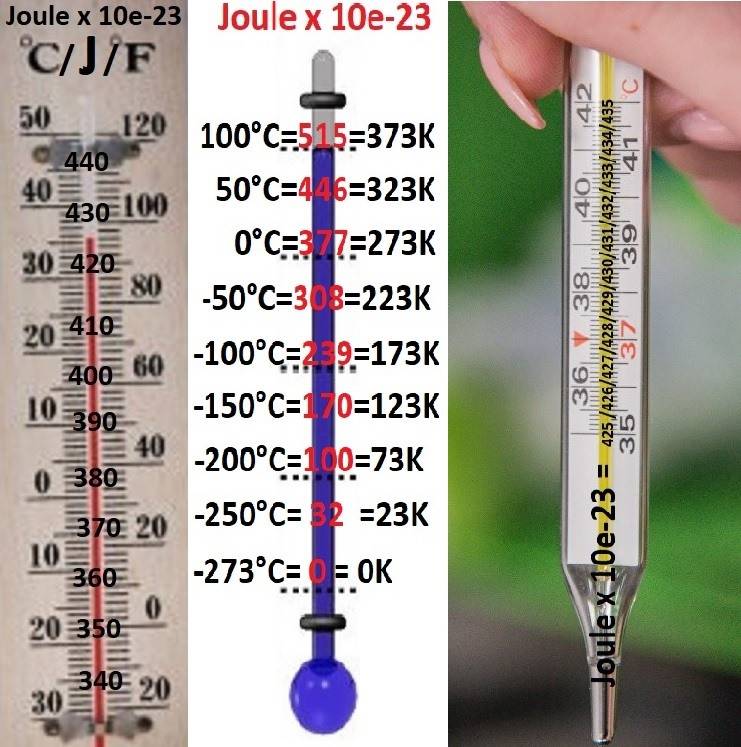
Modern physics believes that kelvin is the basic unit of measurement of SI (one of seven).
At the same time, in all encyclopedias and textbooks it is written:
T= Θ/k = Θ /1,380649x10е-23
Θ - is the energy of the molecule in joules.
k - is the Boltzmann constant = 1,380649e-23 J / K
T - is the absolute temperature in kelvin.
From the formula it follows that the absolute temperature of kelvin is derived through the joules of kinetic energy of the molecule.
And this means that Kelvin is not the basic unit, but a derived unit.
Another proof that kelvin is not the basic, but a derivative, is a site-converter
Convert kelvin [K] <-> joule [J]
https://www.translatorscafe.com/unit-converter/EN/...
2. In accordance with the fact that kelvin is not the basic unit, it is proposed to legalize, along with the use of the Celsius scale and others, the mandatory parallel use of the energy scale in joules.
That is, in addition to “historically established practice”, habits, inertia of thinking, temperature measurement in degrees, not joules, is unjustified.
In addition, the measurement of temperature in degrees (Celsius, Kelvin, Fahrenheit, etc.) hides, disguises the essence of temperature as a physical phenomenon, complicates its understanding and contributes to the physical illiteracy of the people.
THERMOMETERS WITH JOULE - SCALE: