- #1
maistral
- 240
- 17
I have a question about this mixing rule.
Seeing that it allows activity coefficients be used, and seeing that this mixing rule is very, very similar to the Predictive Soave-Redlich-Kwong (PSRK) equation of state's mixing rule, and seeing that other VLE simulators' PSRK VLE graphs are very, very similar to the modified Raoult's law VLE graphs; -
I am having the assumption that any mixing rule that incorporates a local-composition activity coefficient method (NRTL, Wilson, etc etc) in an equation of state method should give almost (if not exactly) the same graph as the one with the modified Raoult's law graph assuming that the ideal gas law is valid at that particular operating pressure.
My problem is that I seem to be unable to generate the same graphs :( I don't know if my equations are incorrect or something, I'm beginning to think that there's something wrong with my partial derivative d(na)/d(n) in the fugacity equations. I hope someone can help me in this, I can't find what's wrong @_@
Details about PSRK are here:
http://en.wikipedia.org/wiki/PSRK
These are the equations I'm using; CEoS = -0.64663.
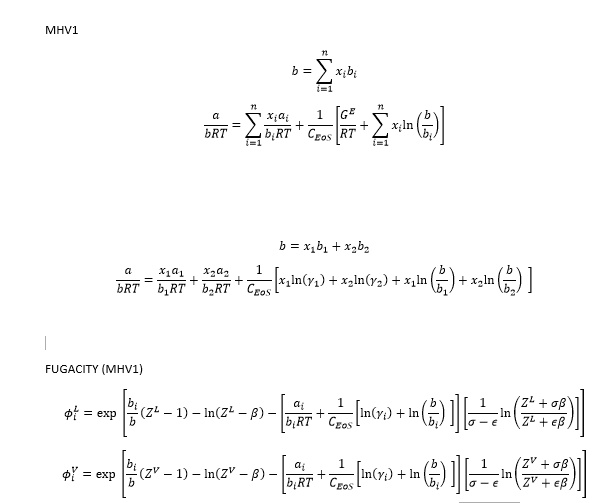
Seeing that it allows activity coefficients be used, and seeing that this mixing rule is very, very similar to the Predictive Soave-Redlich-Kwong (PSRK) equation of state's mixing rule, and seeing that other VLE simulators' PSRK VLE graphs are very, very similar to the modified Raoult's law VLE graphs; -
I am having the assumption that any mixing rule that incorporates a local-composition activity coefficient method (NRTL, Wilson, etc etc) in an equation of state method should give almost (if not exactly) the same graph as the one with the modified Raoult's law graph assuming that the ideal gas law is valid at that particular operating pressure.
My problem is that I seem to be unable to generate the same graphs :( I don't know if my equations are incorrect or something, I'm beginning to think that there's something wrong with my partial derivative d(na)/d(n) in the fugacity equations. I hope someone can help me in this, I can't find what's wrong @_@
Details about PSRK are here:
http://en.wikipedia.org/wiki/PSRK
These are the equations I'm using; CEoS = -0.64663.