- #1
Zohar
- 6
- 1
- TL;DR Summary
- Oxidation state and charge
Hey, y'all.
I know the oxidation state of a carbon in an ethene is -2 while carbon in Acetylene is -1. As well I know acetylene has more disspating elcetrons due to pai bonds. So how come charges between the acetylene carbon are more negative than in ethene while the carbones oxidations states are more negetive on ethene.
My main question is; Is there any connection between oxidation states and molecules charges areas or they are the absulute opposite?
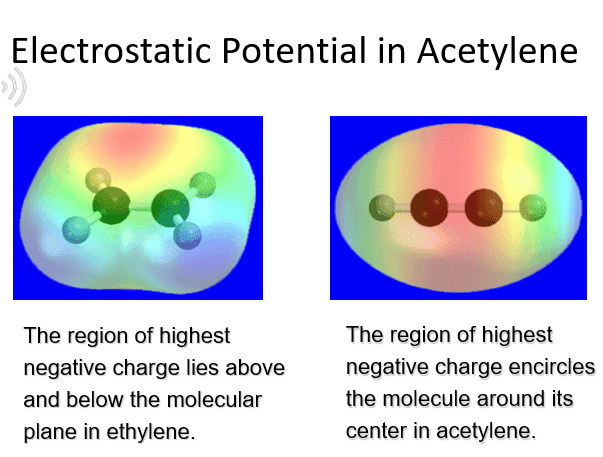
I know the oxidation state of a carbon in an ethene is -2 while carbon in Acetylene is -1. As well I know acetylene has more disspating elcetrons due to pai bonds. So how come charges between the acetylene carbon are more negative than in ethene while the carbones oxidations states are more negetive on ethene.
My main question is; Is there any connection between oxidation states and molecules charges areas or they are the absulute opposite?
Last edited by a moderator: