- #1
Dreak
- 52
- 0
Hello,
In the image below, we see the result of the Zeeman and Paschen-Back effect in a magnetic field which we worked out, together with the exact result, described in different basesets (coupled J,mj and uncoupled mlms).
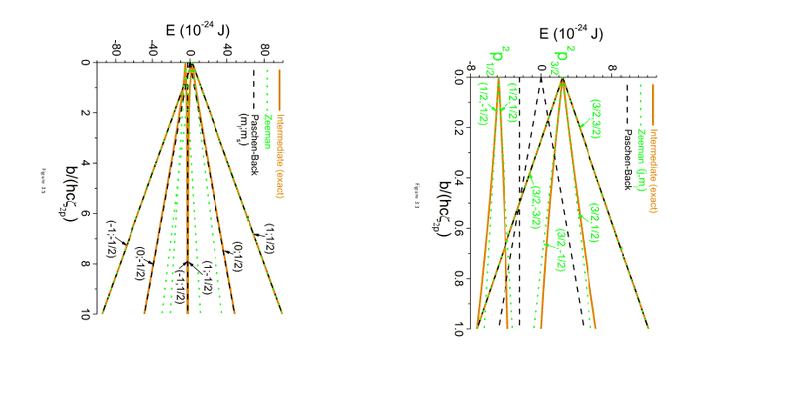
I understand that each effect can be better described in a different set, but I don't understand how that the result can be so 'wrong' compared to the exact result by using this different base.
I thought it was possible to go from coupled to uncoupled and visa versa, doesn't this also mean that an effect has to give the same results in a different base? Where does these errors come from?
I hope my question is clear enough :X
In the image below, we see the result of the Zeeman and Paschen-Back effect in a magnetic field which we worked out, together with the exact result, described in different basesets (coupled J,mj and uncoupled mlms).
I understand that each effect can be better described in a different set, but I don't understand how that the result can be so 'wrong' compared to the exact result by using this different base.
I thought it was possible to go from coupled to uncoupled and visa versa, doesn't this also mean that an effect has to give the same results in a different base? Where does these errors come from?
I hope my question is clear enough :X