- #1
qkfqkekr
- 1
- 0
- Homework Statement
- write down the mechanism of pinacol arrangement of the following diols
- Relevant Equations
- none
I have a question for the first part a), pinacol rearrangement of 1,1-diphenylethane-1,2-diol (did I name that right..?).
How would you know if protonation takes place on the hydroxyl molecule attached to carbon 1 or carbon 2?
My guess is that the hydroxyl group at carbon 1 would be protonated, since leaving of the hydroxyl group (as water) will yield tertiary carbocation, which is stable.
Is this approach correct?
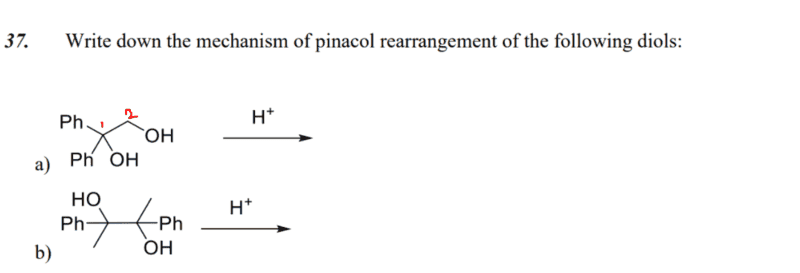
Thank you for your help!
How would you know if protonation takes place on the hydroxyl molecule attached to carbon 1 or carbon 2?
My guess is that the hydroxyl group at carbon 1 would be protonated, since leaving of the hydroxyl group (as water) will yield tertiary carbocation, which is stable.
Is this approach correct?
Thank you for your help!