- #1
phantomvommand
- 242
- 38
- Homework Statement
- Please see pictures and text below
- Relevant Equations
- This is mostly a conceptual question, but intensity distribution equation for diffraction grating may be helpful.
First question: When shining a laser (##\lambda = 630nm##) through a 2D crystal, diffraction patterns are observed. Why?
Possible reasons:
1. Atoms and the space between them act as diffraction gratings.
2. Bragg Diffraction, although in this case the pattern would be observed on the same side as the crystal?
3. X-Ray diffraction. In XRD, are the emitted photons of the excited electrons emitted in random directions? If so, how can a diffraction pattern be seen?
From this part onwards, I have assumed reason 1 to be the reason why such patterns are observed.
Second question: Why are the following diffraction patterns caused by the following crystals? The pictures shown are square unit cells, which make up the respective crystals. Each unit cell of the crystal contains atom(s), which is/are the black square.
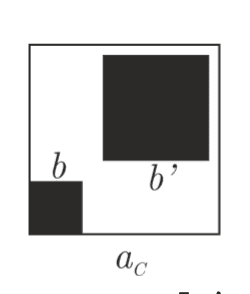

What I am confused about:
- The big atom is displaced vertically from the small atom, so shouldn't the diffraction pattern caused by the big atom be displaced upwards?
Possible reason: The big atom allows so little light to pass through that even the maxima is not visible, due to extremely low intensities, so the entire pattern is caused by the small atom.
- Why is there this "kite" shaped pattern? Without the big atom, the diffraction pattern is a "square" shaped pattern. (see Figure 1 below)
- Would the diagonal of the above crystal form a diffraction grating, although with differing slit width? If so, why is the diffraction pattern of such a grating not visible, or does it overlap with the vertical and horizontal diffraction pattern?
 <-- Figure 1
<-- Figure 1
I also have some questions about why the crystal on the right results in the left pattern.
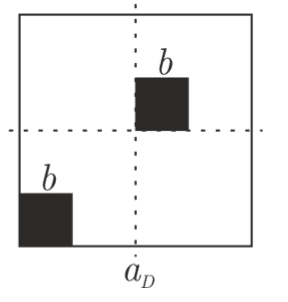

- In this case, 1 of the atoms is displaced upwards and rightwards. To me, it is expected that there is a corresponding displacement in the diffraction pattern. (Contrast with above case)
- If you look very carefully, there are pairs of dots everywhere in the above pattern. What causes them?
Possible reason:
The dots are the secondary maxima of the diffraction grating formed by the horizontal row of atoms. However, why the vertical diffraction grating does not cause these pairs of dots is unclear.
Thank you for reading through such a long post. If anything is unclear, you can refer to: https://onedrive.live.com/?authkey=...!147488&parId=85D7064386310CC9!147487&o=OneUp
for the original problem.
Possible reasons:
1. Atoms and the space between them act as diffraction gratings.
2. Bragg Diffraction, although in this case the pattern would be observed on the same side as the crystal?
3. X-Ray diffraction. In XRD, are the emitted photons of the excited electrons emitted in random directions? If so, how can a diffraction pattern be seen?
From this part onwards, I have assumed reason 1 to be the reason why such patterns are observed.
Second question: Why are the following diffraction patterns caused by the following crystals? The pictures shown are square unit cells, which make up the respective crystals. Each unit cell of the crystal contains atom(s), which is/are the black square.
What I am confused about:
- The big atom is displaced vertically from the small atom, so shouldn't the diffraction pattern caused by the big atom be displaced upwards?
Possible reason: The big atom allows so little light to pass through that even the maxima is not visible, due to extremely low intensities, so the entire pattern is caused by the small atom.
- Why is there this "kite" shaped pattern? Without the big atom, the diffraction pattern is a "square" shaped pattern. (see Figure 1 below)
- Would the diagonal of the above crystal form a diffraction grating, although with differing slit width? If so, why is the diffraction pattern of such a grating not visible, or does it overlap with the vertical and horizontal diffraction pattern?
I also have some questions about why the crystal on the right results in the left pattern.
- In this case, 1 of the atoms is displaced upwards and rightwards. To me, it is expected that there is a corresponding displacement in the diffraction pattern. (Contrast with above case)
- If you look very carefully, there are pairs of dots everywhere in the above pattern. What causes them?
Possible reason:
The dots are the secondary maxima of the diffraction grating formed by the horizontal row of atoms. However, why the vertical diffraction grating does not cause these pairs of dots is unclear.
Thank you for reading through such a long post. If anything is unclear, you can refer to: https://onedrive.live.com/?authkey=...!147488&parId=85D7064386310CC9!147487&o=OneUp
for the original problem.